A complex reaction takes place in following steps.
$$ \begin{aligned} & \mathrm{NO}_2 \mathrm{Cl}_{(\mathrm{g})} \longrightarrow \mathrm{NO}_{2(\mathrm{~g})}+\mathrm{Cl}_{(\mathrm{g})} \text { (slow) } \\ & \mathrm{NO}_2 \mathrm{Cl}_{(\mathrm{g})}+\mathrm{Cl}_{(\mathrm{g})} \longrightarrow \mathrm{NO}_{2(\mathrm{~g})}+\mathrm{Cl}_{2(\mathrm{~g})} \text { (fast) } \end{aligned} $$
Identify rate law equation for this reaction.
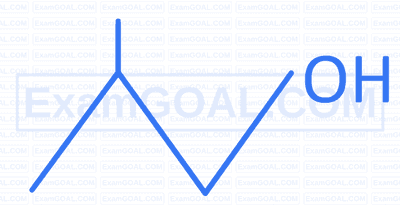
Which among the following has highest boiling point?
Which of the following is correct decreasing order of bond length regarding $\mathrm{N}_2, \mathrm{O}_2$ and $\mathrm{Cl}_2$ ?
Calculate the standard enthalpy change of following reaction.
$$ \begin{aligned} & \mathrm{CH}_{4(\mathrm{~s})}+2 \mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{CO}_{2(\mathrm{~g})}+2 \mathrm{H}_2 \mathrm{O}_{(\ell)} \\ & \text { if } \Delta_{\mathrm{f}} \mathrm{H}^{-}\left(\mathrm{CH}_4\right)=-75 \mathrm{~kJ} \mathrm{~mol}^{-1} \end{aligned} $$
$$ \Delta_{\mathrm{f}} \mathrm{H}^*\left(\mathrm{CO}_2\right)=-390 \mathrm{~kJ} \mathrm{~mol}^{-1} $$
$$ \Delta_{\mathrm{r}} \mathrm{H}^{\circ}\left(\mathrm{H}_2 \mathrm{O}\right)=-286 \mathrm{~kJ} \mathrm{~mol}^{-1} $$