1
MHT CET 2025 23rd April Morning Shift
MCQ (Single Correct Answer)
+1
-0
A weak base is $5 \%$ dissociated in its 0.01 M solution. Calculate the dissociation constant.
2
MHT CET 2025 23rd April Morning Shift
MCQ (Single Correct Answer)
+1
-0
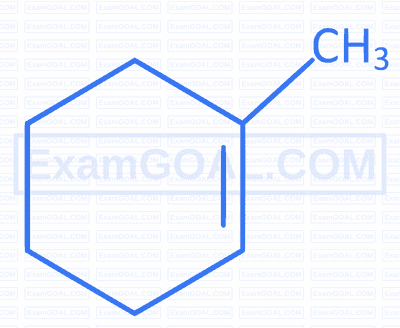
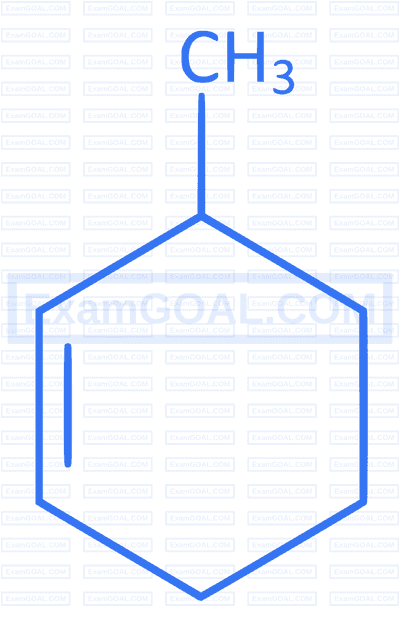
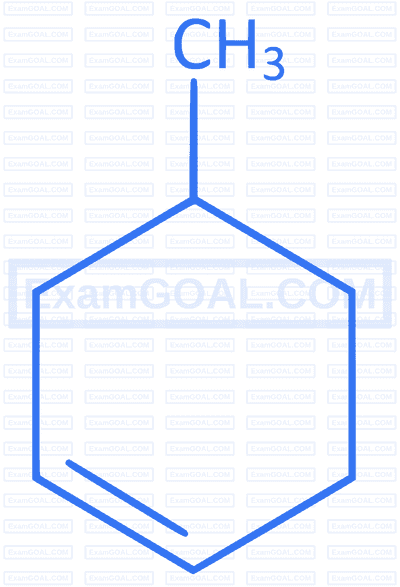
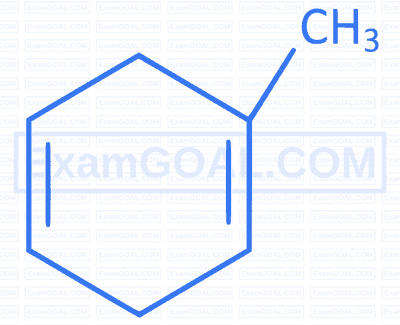
Which of the following compounds forms 1-bromo-1-methylcyclohexane when treated with HBr ?
3
MHT CET 2025 23rd April Morning Shift
MCQ (Single Correct Answer)
+1
-0
$$
\text { Identify substrate ' } S \text { ' in the following reaction. }
$$
$$ \mathrm{S} \xrightarrow{\text { Dimethylcadmium }} \text { Propanone }+ \text { Cadmium chloride } $$
4
MHT CET 2025 23rd April Morning Shift
MCQ (Single Correct Answer)
+1
-0
Calculate work done in isothermal reversible expansion of 1 mole ideal gas from initial pressure 10 bar to final pressure 1 bar at 300 K . $\left(\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right)$.
Paper analysis
Total Questions
Chemistry
50
Mathematics
50
Physics
50
More papers of MHT CET
MHT CET 2025 23rd April Evening Shift
MHT CET 2025 23rd April Morning Shift
MHT CET 2025 22nd April Evening Shift
MHT CET 2025 22nd April Morning Shift
MHT CET 2025 21st April Evening Shift
MHT CET 2025 21st April Morning Shift
MHT CET 2025 20th April Evening Shift
MHT CET 2025 20th April Morning Shift
MHT CET 2025 19th April Evening Shift
MHT CET 2025 19th April Morning Shift
MHT CET 2024 16th May Evening Shift
MHT CET 2024 16th May Morning Shift
MHT CET 2024 15th May Evening Shift
MHT CET 2024 15th May Morning Shift
MHT CET 2024 11th May Evening Shift
MHT CET 2024 11th May Morning Shift
MHT CET 2024 10th May Evening Shift
MHT CET 2024 10th May Morning Shift
MHT CET 2024 9th May Evening Shift
MHT CET 2024 9th May Morning Shift
MHT CET 2024 4th May Evening Shift
MHT CET 2024 4th May Morning Shift
MHT CET 2024 3rd May Evening Shift
MHT CET 2024 3rd May Morning Shift
MHT CET 2024 2nd May Evening Shift
MHT CET 2024 2nd May Morning Shift
MHT CET 2023 14th May Evening Shift
MHT CET 2023 14th May Morning Shift
MHT CET 2023 13th May Evening Shift
MHT CET 2023 13th May Morning Shift
MHT CET 2023 12th May Evening Shift
MHT CET 2023 12th May Morning Shift
MHT CET 2023 11th May Evening Shift
MHT CET 2023 11th May Morning Shift
MHT CET 2023 10th May Evening Shift
MHT CET 2023 10th May Morning Shift
MHT CET 2023 9th May Evening Shift
MHT CET 2023 9th May Morning Shift
MHT CET 2022 11th August Evening Shift
MHT CET 2021 24th September Evening Shift
MHT CET 2021 24th September Morning Shift
MHT CET 2021 23rd September Evening Shift
MHT CET 2021 23th September Morning Shift
MHT CET 2021 22th September Evening Shift
MHT CET 2021 22th September Morning Shift
MHT CET 2021 21th September Evening Shift
MHT CET 2021 21th September Morning Shift
MHT CET 2021 20th September Evening Shift
MHT CET 2021 20th September Morning Shift
MHT CET 2020 19th October Evening Shift
MHT CET 2020 16th October Evening Shift
MHT CET 2020 16th October Morning Shift
MHT CET 2019 3rd May Morning Shift
MHT CET 2019 2nd May Evening Shift
MHT CET 2019 2nd May Morning Shift
MHT CET
Papers
2025
2024
2023
2021
2020