AIPMT 2004
Paper was held on
Sun, May 2, 2004 10:00 AM
Biology
1
When CO2 concentration in blood increases breathing becomes
2
Which one of the following precedes reformation of the nuclear envelope during M phase of the cell cycle?
3
In C3 plants, the first stable product of photosynthesis during the dark reaction is
4
Plants adapted to low light intensity have
5
In glycolysis, during oxidation electrons are removed by
6
Cell elongation in internodal regions of the green plants takes place due to
7
One set of the plant was grown at 12 hours day and 12 hours night period cycles and it flowered while in the other set night phase was interrupted by flash of light and it did not produce flower. Under which one of the following categories will you place this plant?
8
Blood analysis of a patient reveals an unusually high quantity of carboxyhaemoglobin content. Which of the following conclusions is most likely to be correct?
The patient has been inhaling polluted air containing unusually high content of
The patient has been inhaling polluted air containing unusually high content of
9
If you are provided with root-tips of onion in your class and are asked to count the chromosomes, which of the following stages can you most conveniently look into?
10
You are required to draw blood from a patient and to keep it in a test tube for analysis of blood corpuscles and plasma. You are also provided with the following four types of test tubes.
which of these you will not use for the purpose.
which of these you will not use for the purpose.
11
In the resting state of the neural membrane, diffusion due to concentration gradients, if allowed, would drive
12
Injury to vagus nerve in humans is not likely to affect
13
Which one of the following hormones is modified amino acid?
14
Chemically hormones are
15
Which one of the following pairs correctly matches a hormone with a disease resulting from its deficiency?
16
When a diploid female plant is crossed with a tetraploid male, the ploidy of endosperm cells in the resulting seed is
17
An ovule which becomes curved so that the nucellus and embryo sac lie at right angles to the funicle is
18
Anthesis is a phenomenon which refers to
19
Diversification in plant life appeared
20
Which of the following statements is not true for retroviruses?
21
Phenetic classification of organisms is based on
22
A free living nitrogen-fixing cyanobacterium which can also symbiotic association with the water ferm Azolla is
23
Viruses that infect bacteria multiply and cause their lysis, are called
24
Lichens are well known combination of an alga and a fungus where fungus has
25
During replication of a bacterial chromosome DNA synthesis starts from a replication origin site and
26
Ovulation in the human female normally takes
place during the menstrual cycle -
27
Angiosperms have dominated the land flora primarily because of their
28
In Arthropoda, hend and thorax are often used to form cephalothorax, but in which one of the following classes, is the body divided into head thorax and abdomen?
29
The animals with bilateral symmetry in young stage, and radial pentamerous symmetry in the adult stage, belong to the Phylum
30
Presence of gills in the tadpole of frog indicates that
31
Uricotelism is found in
32
When a fresh-water protozoan possessing a contractile vacuole, is placed in a glass containing marrine water, the vacuole will
33
One of the following is a very unique feature of the mammalian body
34
Edible part of mango is
35
Which one of the following enzymes, is copper necessarily associated as an activator?
36
In the somatic cell cycle
37
Which of the following is expected to have the highest value (gm/m2/yr) in a grassland
ecosystem ?
38
Which one of the following pairs is not correctly matched ?
39
Restriction endonucleases :-
40
In transgenics expression of transgene in target
tissue is determined by :-
41
DNA fingerprinting refers to :-
42
The Ti plasmid is often used for making
transgenic plants. This plasmid is found in
43
ELISA is used to detect viruses, wherethe key reagent is :-
44
Certain characteristic demographic features of
developing countries are -
45
What is a keystone species ?
46
In which of the following pairs is the specific
characteristic of a soil not correctly matched ?
47
The maximum growth rate occurs in :-
48
In which one of the following habitats does the
diurnal temperature of soil surface vary most ?
49
The most thoroughly studied of the known
bacteria-plant interactions is the :-
50
An ecosystem which can be easily damaged but
can recover after some time if damaging effect
stops will be having -
51
In your opinion, which is the most effective way
to conserve the plant diversity of an area ?
52
In a longitudinal section of a root, starting from
the tip upward, the four zones occur in the
following order :-
53
Mast cells of connective tissue contain -
54
The telomeres of eukaryotic chromosomes
consist of short sequences of
55
In chloroplasts, chlorophyll is present in the :-
56
A major component of gobar gas is
57
The following ratio is generally constant for a
given species :-
58
Which of the following hormones is not a
secretion product of human placenta -
59
In a plant, red fruit (R) is dominant over yellow
fruit (r) and tallness (T) is dominant over
shortness (t). If a plant with RRTt genotype is
crossed with a plant that is rrtt
60
A male human is heterozygous for autosomal
genes A and B and is also hemizygous for
hemophilic gene h. What proportion of his
sperms will be abh :-
61
Lack of independent assortment of two genes A
and B in fruit fly Drosophila is due to :-
62
One of the parents of a cross has a mutation in
its mitochondria. In that cross, that parent is
taken as a male. During segregation of F2
progenies that mutation is found in -
63
A normal woman, whose father was colour-blind
is married to a normal man. The sons would be :-
64
The recessive genes located on X-chromosomes
in humans are always-
65
After a mutation at a genetic locus the character
of an organism changes due to the change in :-
66
During transcription, if the nucleotide sequence
of the DNA strand that is being coded is
ATACG, then the nucleotide sequence in the
mRNA would be -
67
Which form of RNA has a structure resembling
clover leaf ?
68
In a mutational event, when adenine is replaced
by guanine, it is a case of -
69
Age of fossils in the past was generally
determined by radio-carbon method and other
methods involving radioactive elements found in
the rocks. More precise methods, which were
used recently and led to the revision of the
evolutionary periods for different groups of
organisms includes -
70
What kind of evidence suggested that man is
more closely related with chimpanzee than with
other hominoid apes ?
71
According to oparin, which one of the following
was not present in the primitive atmosphere of
the earth ?
72
Which one of the following is living fossil ?
73
Diversification in plant life appeared
74
Which one of the following is not correctly
matched ?
75
Dough kept overnight in warm weather
becomes soft and spongy because of :-
Chemistry
1
CN$$-$$ is a strong field ligand. This is due to the fact that
2
Which of the following does not have a metal carbon bond?
3
In an octahedral structure, the pair of $$d$$ orbitals involved in d2sp3 hybridisation is
4
Which of the following is considered to be an anticancer species?
5
Considering H2O as a weak field ligand, the number of unpaired electrons in [Mn(H2O)6]2+ will be (atomic number of Mn = 25)
6
The rate of a first order reaction is 1.5 $$ \times $$ 10$$-$$2 mol L$$-$$1 min$$-$$1 at 0.5 M concentration of the reactant. The half-life of the reaction is
7
In a reaction of aniline a coloured product C was obtained.

The structure of C would be

The structure of C would be
8
The hormone that helps in the conversion of glucose to glycogen is
9
The correct statement in respect of protein haemoglobin is that it
10
Number of chiral carbons in $$\beta $$-$$D$$-(+) glucose is
11
A sequence of how many nucleotides in messenger RNA makes a codon for an amino acid?
12
The helical structure of protein is stablised by
13
Which of the following structures represents the peptide chain?
14
The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at 25oC. The equilibrium constant of the reaction would be
15
Which one of the following can be oxidised to the corresponding carbonyl compound?
16
Which one of the following statements about the zeolite is false?
17
Among K, Ca, Fe and Zn, the element which can from more than one binary compound with chlorine is
18
Among the following series of transition metal ions, the one where all metal ions have 3d2 electronic configuration is
[At. Nos. Ti = 22, V = 23, Cr = 24, Mn = 25]
[At. Nos. Ti = 22, V = 23, Cr = 24, Mn = 25]
19
lanthanoids are
20
The radioactive isotope $${}_{27}^{60}$$Co which is used in the treatment of cancer can be made by (n.p) reaction. For this reaction the tarfet nucleus is
21
Which of the following coordination compounds would exhibit optical isomerism?
22
Among [Ni(CO)4], [Ni(CN)4]2$$-$$, [NiCl4]2$$-$$ species, the hybridisation states at the Ni atom are, respectively
[Atomic number of Ni = 28]
[Atomic number of Ni = 28]
23
Considering entropy (S) as a thermodynamic parameter, the criterion for the spontaneity of any process is
24
The maximum number of molecules is present in
25
The frequency of radiation emitted when the electron falls from n = 4 to n = 1 in hydrogen atom will be (Given ionization energy of H = 2.18 $$ \times $$ 10$$-$$18 J atom$$-$$1 and h = 6.625 $$ \times $$ 10$$-$$34 J s)
26
Ionic radii are
27
In a regular octahedral molecule, MX6 the number of X $$-$$ M $$-$$ X bonds at 180o is
28
H2O is dipolar, whereas BeF2 is not. It is because
29
Among the following, the pair in which the two species are not isostructural is
30
In BrF3 molecule, the lone pairs occupy equatorial positions to minimize
31
The work done during the expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atm is (1 L atm = 101.32 J)
32
Standard enthalpy and standard entropy changes for the oxidation of ammonia at 298 K are $$-$$ 382.64 kJ mol$$-$$1 and $$-$$ 145.6 kJ mol$$-$$1, respectively. Standard Gibb's energy change for the same reaction at 298 K is
33
If the bond energies of H $$-$$ H, Br $$-$$ Br, and H $$-$$ Br are 433, 192 and 364 kJ mol$$-$$1 respectively, the $$\Delta $$Ho for the reaction
H2(g) + Br2(g) $$ \to $$ 2HBr(g) is
H2(g) + Br2(g) $$ \to $$ 2HBr(g) is
34
The solubility product of a sparingly soluble salt AX2 is 3.2 $$ \times $$ 10$$-$$11. Its solubility (in moles/L) is
35
The rapid change of pH near the stoichiometric point of an acid-base titration is the basis of indicator detection. pH of the solution is related to ratio of the concentrations of the conjugate acid (HIn) and base (In$$-$$) forms of the indicator by the expression
36
The $$-$$ OH group of an alcohol or the $$-$$ COOH group of a carboxylic acid can be replaced by $$-$$ Cl using
37
The molecular formula of diphenyl methane,

How many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom ?

How many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom ?
38
Using anhydrous AlCl3 as catalyst, which one of the following reactions produces ethylbenzene (PhEt)?
39
Reaction of HBr with propene in the presence of peroxide gives
40
Which of the following is least reactive in a nucleophilic sibstitution reaction?
41
Which one of the following will not form a yellow precipitate on heating with an alkaline solution of iodine?
42
Which is the best description of the behaviour of bromine in the reaction given below?
H2O + Br2 $$ \to $$ HOBr + HBr
H2O + Br2 $$ \to $$ HOBr + HBr
Physics
1
The refractive index of the material of a prism is $$\sqrt 2 $$ and its refracting angle is 30o. One of the refracting surfaces of the prism is made a mirror inwards. A beam of monochromatic light entering the prism from the other face with retrace its path after reflection from the mirrored surface if its angle of incidence on the prism is
2
Five equal resistances each of resistances R are connected as shown in the figure. A battery of V volts is connected between A and B. The current flowing in AFCEB will be
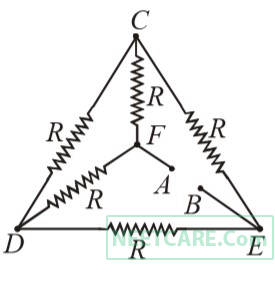

3
when three identical bulbs of 60 watt, 200 volt rating are connected in series to a 200 volt supply, the power drawn by them will be
4
The electric resistance of a certain wire of iron is R. If its length and radius are both doubled, then
5
In India electricity is supplied for domestic use at 220 V. It is supplied at 110 V in USA. If the resistance of a 60 W bulb for use in India is R, the resistance of a 60 W bulb for use in USA will be
6
Resistance n, each of r ohm, when connected in parallel give an equivalent resistance of R ohm. If these resistances were connected in series, the combination would have a resistance in ohms, equal to
7
A 6 volt battery is connected to the terminals of a three metre long wire of uniform thickness and resistance of 100 ohm. The difference of potential between two points on the wire separated by a distance of 50 cm will be
8
A galvanometer of 50 ohm resistance has 25 divisions. A current of 4 $$ \times $$ 10$$-$$4 ampere gives a deflection of one division. To convert this galvanometer into a voltmeter having a range of 25 volts, it should be connected with a resistance of
9
To convert a galvanometer into a voltmeter one should connect a
10
A coil of 40 henry inductance is connected in series with a resistance of 8 ohm and the combination is joined to the terminals of a 2 volt battery. The time constant of the circuit is
11
The magnetic flux through a circuit of resistance R changes by an amount $$\Delta $$$$\phi $$ in a time $$\Delta $$t. Then the total quantity of electric charge Q that passes any point in the circuit during the time $$\Delta $$t is represented by
12
A telescope has an objective lens of 10 cm diameter and is situated at a distance of one kilometer from two objects. The minimum distance between these two objects, which can be resolved by the telescope, when the mean wavelength of light is 5000 $$\mathop A\limits^ \circ $$, is of the order of
13
An electric dipole has the magnitude of its charge as q and its dipole moment is p. It is placed in a uniform electric field E. If its dipole moment is along the direction of the field, the force on it and its potential energy are respectively
14
A beam of light composed of red and green ray is incident obliquely at a point on the face of rectangular glass slab. When coming out on the opposite parallel face, the red green ray emerge from
15
According to Einstein's photoelectric equation, the graph between the kinetic energy of photoelectrons ejected and the frequency of incident radiation is
16
If M(A; Z), Mp and Mn denote the masses of the nucleus $${}_Z^AX,$$ proton and neutron respectively in units of u (1 u = 931.5 MeV/c2) and BE represents its bonding energy in MeV, then
17
If in a nuclear fusion process the masses of the fusing nuclei be m1 and m2 and the mass of the resultant nucleus be m3, then
18
The Bohr model of atoms
19
The peak voltage in the output of a half wave diode rectifier fed with a sinusoidal signal without filter is 10 V. The d.c. component of the output voltage is
20
In semiconductors at a room temperature
21
Of the diodes shown in the following diagrams, which one is reverse biased ?
22
A round disc of moment of inertia $$I$$2 about its axis perpendicular to its plane and passing through its centre is placed over another disc of moment of inertia $$I$$1 rotating with an angular velocity $$\omega $$ about the same axis. The final angular velocity of the combination of discs is
23
The unit of permittyvity of free space, $${\varepsilon _0}$$, is
24
If $$\left| {\overrightarrow A \times \overrightarrow B } \right| = \sqrt 3 \overrightarrow A .\overrightarrow B $$ then the value of $$\left| {\overrightarrow A + \overrightarrow B } \right|$$ is
25
A block of mass m is placed on a smooth wedge of inclination $$\theta $$. The whole system is accelerated horizontally so that the block does not slip on the wedge. The force exerted by the wedge on the block will be (g is acceleration due to gravity)
26
The coefficient of static friction, $$\mu $$s, between block A of mass 2 kg and the table as shown in the figure is 0.2. What would be the maximum mass value of block B so that the two blocks do not move? The string and the pulley are assumed to be smooth and massless. (g = 10 m/s2)


27
A ball of mass 2 kg and another of mass 4 kg are dropped together from a 60 feet ball building. After a fall of 30 feet each towards earth, their respective kinetic energies will be in the ratio of
28
A particle of mass m1 is moving with a velocity v1 and another particle of mass m2 is moving with a velocity v2. Both of them have the same momentum but their different kinetic energies are E1 and E2 respectively. If m1 > m2 then :
29
A mass of 0.5 kg moving with a speed of 1.5 m/s on horizontal smooth surface, collides with a nearly weightless spring of force constant k = 50 N/m. The maximum compression of the spring would be


30
Three particles, each of mass m gram, are situated at the vertices of an equilateral triangle ABC of side $$l$$ cm (as shown in the figure). The moment of inertia of the system about a line AX perpendicular to AB and in the plane of ABC, in gram-cm2 units will be


31
A wheel having moment of inertia 2 kg m2 about its vertical axis, rotates at the rate of 60 rpm about this axis. The torque which can stop the wheel's rotation in one minute would be
32
Consider a system of two particles having masses m1 and m2. If the particle of mass m1 is pushed towards the mass centre of particles through a distance d. by what distance would be particle of mass m2 move so as to keep the mass centre of particles at the original position?
33
The output of OR gate is 1
34
The ratio of the radii of gyration of a circular disc about a tangential axis in the plane of the disc and of a circular ring of the same radius about a tangential axes in the plane of the ring is
35
The density of a newly discovered planet is twice that of earth. The acceleration due to gravity at the surface of the planet is equal to that at the surface of the earth. If the radius of the earth is R, the radius of the planet would be
36
If $$\lambda $$m denotes the wavelength at which the radioactive amission from a black body at a temperature TK is maximum, then
37
The equation of state for 5 g of oxygen at a pressure P and temperature T, when occupying a volume V, will be
(where R is the gas constant)
(where R is the gas constant)
38
One mole of an ideal gas at an initial temperature of T K does 6R joule of work adiabatically. If the ratio of specific heats of this gas at constant pressure and at constant volume is 5/3, the final temperature of gas will be
39
Two springs of spring constants k1 and k2 are joined in series. The effective spring constant of the combination is given by
40
A particle executing simple harmonic motion of amplitude 5 cm has maximum speed of 31.4 cm/s. The frequency of its oscillation is
41
The phase difference between two waves. represented by
y1 = 10$$-$$6 sin[100t + (x/50) + 0.5] m
y2 = 10$$-$$6 cos[100t + (x/50)] m,
where x is expressed in metres and t is exressed in secondss, is approximately.
y1 = 10$$-$$6 sin[100t + (x/50) + 0.5] m
y2 = 10$$-$$6 cos[100t + (x/50)] m,
where x is expressed in metres and t is exressed in secondss, is approximately.
42
A bullet of mass 2 g is having a charge of 2 $$\mu $$C. Through what potential difference must it be accelerated, starting from rst, to acquire a speed of 10 m/s ?
43
The dimensions of universal gravitational constant are