Chemistry
Arrange the complex ions in the increasing order of their magnetic moments
A. $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$
B. $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}$
C. $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
D. $\left[\mathrm{FeF}_6\right]^{3-}$
Two statements, one Assertion and the other Reason, are given. Choose the correct option.
Assertion : During the electrolysis of aqueous $\mathrm{NaCl}, \mathrm{Cl}_2$ is liberated at the anode in preference to $\mathrm{O}_2$ and water gets reduced to $\mathrm{H}_2$ at cathode.
Reason : The reaction at Anode with lower oxidation potential is not preferred due to over potential of Oxygen.
Identify the correct coefficients (a), (b), (c) and (d) in the following equations
i) $\quad \mathrm{xMnO}_4^{--}+$(a) $\mathrm{SO}_3{ }^{2-}+$ (b) $\mathrm{H}^{+} \cdots \longrightarrow \mathrm{xMn}^{2+}+$ (a) $\mathrm{SO}_4{ }^{2-}+$ (c) $\mathrm{H}_2 \mathrm{O}$
ii) (c) $\mathrm{S}_2 \mathrm{O}_3{ }^{2-}+$ (d) $\mathrm{MnO}_4^{-}+\mathrm{H}_2 \mathrm{O} \cdots \rightarrow$ (d) $\mathrm{MnO}_2+$ (b) $\mathrm{SO}_4{ }^{2-}+\mathrm{xOH}^{-}$
For a reaction,
$$A+B \rightleftharpoons 2 C$$
1.0 mole of $A, 1.5$ mole of $B$ and 0.5 mole of $C$ were taken in a 1 L vessel. At equilibrium, the concentration of $C$ was $1.0 \mathrm{~mol} \mathrm{~L}^{-1}$. The equilibrium constant for the reaction is $x / 15$. The value of ' $x$ ' is:
Match the reactions given in Column I with the major product formed given in Column II.
| S.No. | Reactions | S.No. | Major product formed |
|---|---|---|---|
| A. |  |
P. |  |
| B. |  |
Q. | 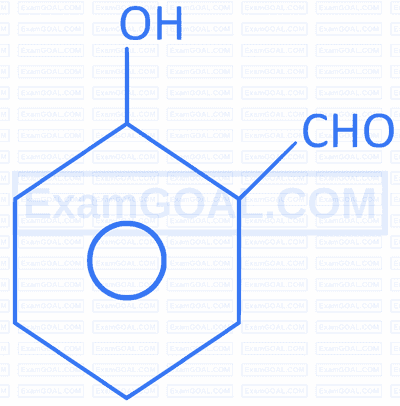 |
| C. | 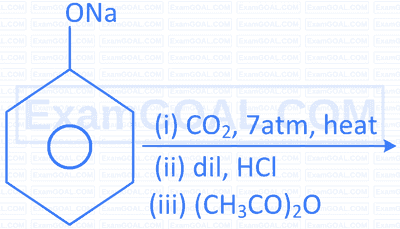 |
R. | 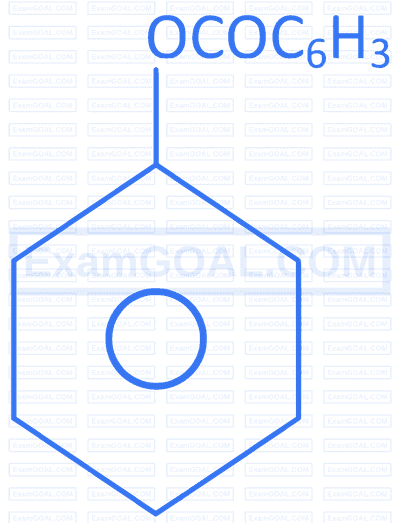 |
| D. | 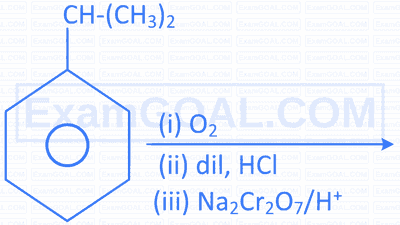 |
S. | 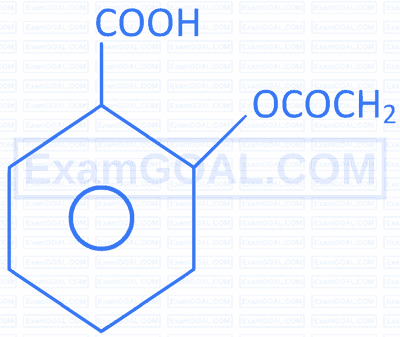 |
Two statements, one Assertion and the other Reason are given. Choose the right option.
Assertion : Insulin is called a protein whereas Glycyl alanine is not called a protein
Reason : A polypeptide with amino acid residue less than 100 can also be called as a protein if it has a well-defined conformation of a protein.
Choose the correct metal/ ion from the brackets which -------------------------
A. has chemical reactivity similar to that of the first few members of the Lanthanoids $(\mathrm{Zn}, \mathrm{Ca}, \mathrm{Fe}, \mathrm{Cu})$.
B. has stable $4 \mathrm{f}^7$ electronic configuration, but acts as a strong reducing agent and converts to $\mathrm{M}^{3+}$ state. $\left(\mathrm{Eu}^{2+}, \mathrm{Ce}^{2+}, \mathrm{Pr}^{2+}, \mathrm{Dy}^{2+}\right)$
C. is a colorless ion $\left(\mathrm{Tm}^{3+}, \mathrm{Lu}^{3+}, \mathrm{Gd}^{3+}, \mathrm{Sm}^{3+}\right)$.
D. shows stable +2 oxidation state and is diamagnetic ( $\mathrm{Ce}, \mathrm{Sm}, \mathrm{Ho}, \mathrm{Yb}$ )
What would be the major product formed in the reaction?

When $0.1 \mathrm{~mol} \mathrm{~L}^{-1}$ of KCl was filled in a Conductivity cell the resistance was 80 ohms at 298 K . (Conductivity of 0.1 M KCl at 298 K is $1.29 \mathrm{~S} \mathrm{~m}^{-1}$. The same cell when filled with an unknown electrolyte of concentration 0.025 M , had a resistance of 92 ohms. What is the Molar conductivity of the electrolyte at the given concentration?
Consider the reaction
$$\mathrm{Fe}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{CO}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{Fe}(\mathrm{l})+3 \mathrm{CQ}_2(\mathrm{~g})$$
In accordance with Le-Chatlier's principle, which of the following will not disturbs the equilibrium?
Identify the final product $[\mathrm{Y}]$ in the following reaction.

Find the correct matches.
| A. | $\mathrm{Cr}_2 \mathrm{O}_7^{2-}$ | P. | is predominantly a basic compound. |
|---|---|---|---|
| B. | $\mathrm{FeCr}_2 \mathrm{O}_4$ | Q. | is predominantly a acidic compound |
| C. | $\mathrm{CrO}_3$ | R. | forms yellow chromate on reaction with $\mathrm{Na}_2 \mathrm{CO}_3$ in air. |
| D. | $\mathrm{CrO}$ | S. | is interconvertible with $\mathrm{MO}_4^{2-}$ depending on the pH of the medium. |
Identify the two incorrect electrochemical reactions shown as taking place in the given cells at the respective electrodes.
| Cell | Electrode | Reaction |
|---|---|---|
| Lead-Storage Cell | Cathode | $\mathrm{Pb}+2 \mathrm{SO}_4{ }^{2-}+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{PbSO}_4+2 \mathrm{H}_2 \mathrm{O}+\mathrm{SO}_2$ |
| Leclanche Cell | Cathode | $\mathrm{MnO}_2+\mathrm{NH}_4^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{MnO}+\mathrm{OH}+\mathrm{NH}_3$ |
| Mercury Cell | Anode | $\mathrm{Zn} / \mathrm{Hg}+2 \mathrm{OH}^{-} \rightarrow \mathrm{ZnO}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-}$ |
| Fuel Cell | Cathode | $\mathrm{O}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-}$ |
Consider the following reaction
$$\text { Propyne }+\mathrm{CH}_3 \mathrm{MgBr} \xrightarrow{\text { dry ether }} \mathbf{X}$$
Identify ' X ' from the following.
Match the IUPAC names in Column II with the correct structures given in Column I.
| Structures of compounds | IUPAC name | ||
|---|---|---|---|
| A | 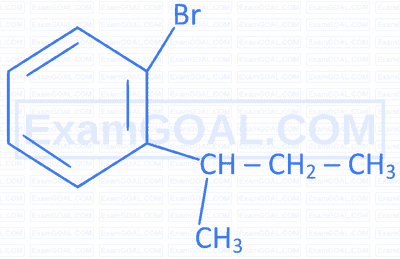 |
P | 1-Bromomethyl-3-(2,2-dimethylpropyl)benzene |
| B | 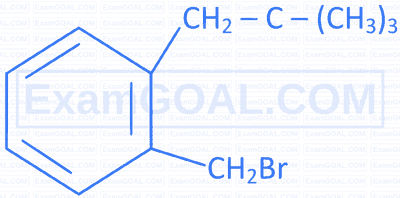 |
Q | 1-Bromo-4-(1-methylethyl)benzene |
| C |  |
R | 1-Bromo-2-(1-methylpropyl)benzene |
| D |  |
S | 1-Bromo-4-(2-methylpropyl)benzene |
Which of the coordination compounds [(i) to (v)] are used for the following processes :
A: Electroplating
B: Removal of excess copper from human body
C: Estimation of hardness of water
(i) DMG (ii) $\mathrm{Na}_2 \mathrm{EDTA}$ (iii) Cis-platin (iv) D-penicillamine (v) $\mathrm{K}\left[\mathrm{Au}(\mathrm{CN})_2\right]$
Identify the type of reaction:

Identify X, Y and Z formed in the reaction:

Identify the Carbonyl compound which will not be formed when hydration of Alkynes is carried out with dil. $\mathrm{H}_2 \mathrm{SO}_4 / \mathrm{Hg}^{2+}$ at 333 K .
Acetone, Butanal, Ethanal, Butanone.
Identify $[\mathrm{X}]$ used in the given reaction.
$[\mathrm{X}]+$ Copper $/ 573 \mathrm{~K} \rightarrow[\mathrm{Y}]$
$[\mathrm{Y}] \xrightarrow[\text { (ii) } \mathrm{H}_2 \mathrm{O} / \mathrm{H}^{+}]{\text {(i) } \mathrm{CH}_3 \mathrm{MgBr}}$ Tert.butyl alcohol. (major product)
Which one of the following is the product $(\mathrm{Z})$ formed at the end of the given reaction:
$$\begin{aligned} & \text { Propan-1-ol }+\mathrm{SOCl}_2 \rightarrow[\mathrm{X}] \\ & {[\mathrm{X}]+\mathrm{HC} \equiv \mathrm{C}-\mathrm{Na} \rightarrow[\mathrm{Y}]} \\ & {[\mathrm{Y}]+\mathrm{H}_2 \mathrm{SO}_4 / \mathrm{HgSO}_4, 330 \mathrm{~K} \rightarrow[\mathrm{Z}]} \end{aligned}$$
Two statements, one Assertion and the other Reason are given. Identify the correct option
Assertion : Primary and secondary amides on treatment with $\mathrm{Br}_2$ and alcoholic NaOH yield primary and secondary amines respectively and the reaction involves stepping down the series.
Reason : The reaction occurs due to the migration of alkyl group from Carbonyl carbon atom to the Nitrogen atom with elimination of carbonyl group as the carbonate salt.
What are the intermediates formed during the reactions $A$ and $B$ ?
A. Reimer-Tiemann reaction.
B. Dehydration of alcohols in the slow rate determining step.
Arrange the following in the increasing order of their covalent character.
$\mathrm{CaF}_2 ; \mathrm{CaCl}_2 ; \mathrm{CaBr}_2 ; \mathrm{CaI}_2$
Mathematics
A function $f$ from the set of natural numbers to integers defined by
$$f(n)=\left\{\begin{array}{l} \frac{n-1}{2}, \quad \text { when } n \text { is odd } \\ -\frac{n}{2}, \quad \text { when } n \text { is even } \end{array} \quad\right. \text { is }$$
Kiran purchased 3 pencils, 2 notebooks and one pen for ₹41. From the same shop Manasa purchased 2 pencils, one notebook and 2 pens for ₹ 29 , while Shreya purchased 3 pencils, 2 notebooks and 2 pens for ₹ 44. The above situation can be represented in matrix form as $A X=B$. Then $|\operatorname{adj} A|$ is equal to
The relationship between a and b for the continuous function
$f(x)=\left\{\begin{array}{ll}a x+1, & \text { if } x \leq 3 \\ b x+3, & \text { if } x>3\end{array}\right.$ at $x=3$ is
Physics
Which of the following is correct in the case of the Bohr model of atoms?
A. Predicts continuous emission spectra for all atoms
B. Assumes that the angular momentum of electrons is quantised
C. Predicts same emission spectrum for singly ionised neon atom and hydrogen atom
D. Predicts same emission spectrum for singly ionised neon atom and singly ionised helium atom
The zener voltage in the circuit shown is $\mathrm{V}_{\mathrm{Z}}=20 \mathrm{~V}$. The load resistance $\mathrm{R}_{\mathrm{L}}=5 \mathrm{k} \Omega$ and the resistance $\mathrm{R}_{\mathrm{S}}=10 \mathrm{k} \Omega$. If the input voltage is $\mathrm{V} \mathrm{s}=100 \mathrm{~V}$, then the current through the zener diode in milliampere is:
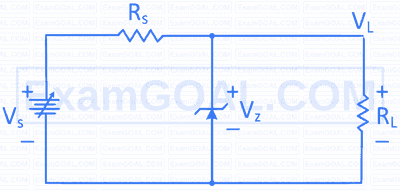
The total number of degrees of freedom associated with $2 \mathrm{~cm}^3$ of Nitrogen gas at normal temperature and pressure is:
[Given Avogadro number as ' N ' ]
Three charges, $Q,-q$ and $2 q$ are placed at the vertices of a right-angled isosceles triangle. What is the value of $q$ for the net electrostatic energy of the configuration to be zero?

If the distance between the Sun and Earth is doubled, then the duration of the year on earth will be :
[Given actual duration of the year $=\mathbf{T}$ ]
A network of capacitors is as shown below. If the voltage supply is 100 V , find the energy stored in the $6 \mu \mathrm{~F}$ capacitor.

$C_1=3 \mu F, C_2=6 \mu F, C_3=3 \mu F$ and $C_4=4 \mu F$
The power dissipated across the $16 \Omega$ resistor in the circuit is 2 watts. The power dissipated in watt units across the $4 \Omega$ resistor is:

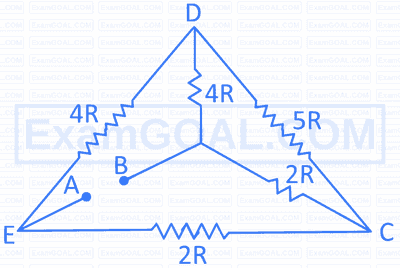
The net resistance between the points A and B in the circuit given below is:
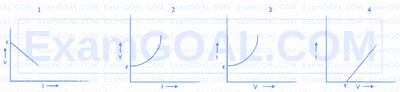
A student measures the terminal potential difference $V$ of a cell of emf $\varepsilon$ and internal resistance $r$ as a function of the current I flowing through it. Which of the following graphs will give the values of emf $\varepsilon$ and internal resistance $r$ ?
A plot of kinetic energy of emitted photoelectrons from a metal versus the frequency of incident radiation gives a straight line, the intercept of which
A. Depends on the nature of the metal used
B. Depends on the intensity of radiation
C. Depends both on the intensity and the nature of metal used
D. Is a constant and is same for all metals which is independent of the intensity of Incident radiation
A current carrying closed loop in the form of a right isosceles triangle XYZ is placed in a uniform magnetic field $B$ acting along $X Y$ of the loop. If the magnetic force on the arm $Y Z$ is $\sqrt{2} F$, then the force on the arm $X Z$ is:
