Chemistry
Identify the final product $[Z]$ formed when Chlorobenzene undergoes the given series of reactions:

Match the metals in Column II with their characteristic properties given in Column I.
| Properties | Metals | ||
|---|---|---|---|
| A. | Lanthanoid with low value of $3^{\text {rd }}$ ionisation enthalpy. | P | V |
| B. | f-Block element with electronic configuration $[R n] 6 d^2 7 s^2$ | Q | Gd |
| C. | 3 d -series metal whose $\mathrm{M}^{3+}{ }_{(\mathrm{aq})}$ is colourless. | R | Th |
| D. | 3d-series metal with highest $\Delta \mathrm{H}_{\text {(atomisation) }}$ | S | Sc |
Convert Benzene $\rightarrow 3$-Bromophenol by choosing appropriate reagents [(i) to (v)] in the correct sequence.
(i) $\mathrm{NaNO}_2 / \mathrm{HCl}\left(0^{\circ} \mathrm{C}\right)$
(ii) Conc. $\mathrm{HNO}_3 / \mathrm{H}_2 \mathrm{SO}_4$
(iii) $\mathrm{H}_2 \mathrm{O} / 283 \mathrm{~K}$
(iv) $\mathrm{Fe} / \mathrm{HCl}$
(v) $\mathrm{Br} 2 / \mathrm{Fe}$
Two statements, One Assertion (A) and the other Reason (R) are given. Choose the right option.
Assertion: The rate constant $(\mathrm{k})$ for a chemical reaction gets nearly doubled for a $10^0$ rise in temperature.
Reason: The number of bimolecular collisions between reactant molecules increase with increase in temperature
What is the final product $[\mathrm{Z}]$ formed when the given reactions take place?

Two statements, one Assertion (A) and the other Reason (R) are given. Choose the correct option.
Assertion: Compound $[\mathrm{X}]$ reacts with Hydrazine in presence of $\mathrm{KOH} /$ Glycol to form the product $[\mathrm{Y}]$.

Reason: Reduction reaction occurs and Carbonyl group is reduced to Methylene group.
$$\mathrm{E}^0\left(\mathrm{Fe}^{3+} / \mathrm{Fe}^{2+}\right)=0.771 \mathrm{~V}$$
Both reactions (i) and (ii) give the same compound X as the major product. Identify X
(i). 3-Methylbut-1-ene $+\mathrm{HCl} \rightarrow \mathrm{X}$
(ii). Neopentyl alcohol $+\mathrm{HCl}\left(\right.$ anh. $\left.\mathrm{ZnCl}_2\right) \rightarrow \mathrm{X}$
Arrange the following compounds in the decreasing order of the molar conductivities of their aqueous solutions.
| A | B | C | D |
|---|---|---|---|
| $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{Cl}_2$ | $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]$ | $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right] \mathrm{Cl}$ | $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3$ |
From the given covalent compounds (A to F) identify the pair of molecules which have:
(i) Two lone pairs of electrons on the central atom.
(ii) One lone pair of electrons on the central atom.
A. $\mathrm{SO}_2$
B. $\mathrm{ClF}_3$
C. $\mathrm{BF}_3$
D. $\mathrm{BrF}_5$
E. XeF 4
F. $\mathrm{SF}_6$
Arrange the following free radicals in the increasing order of their stability:
| A | B | C | D |
|---|---|---|---|
 |
 |
 |
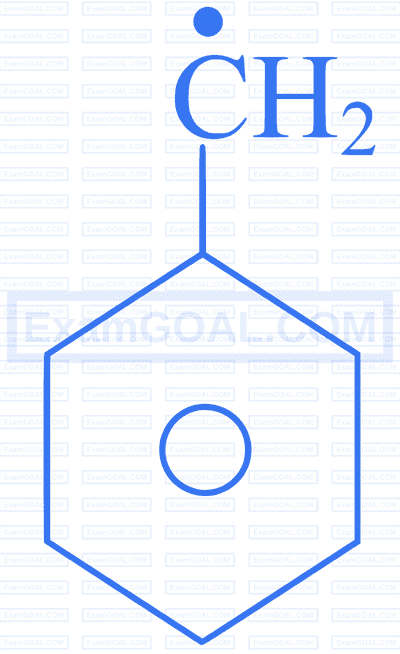 |
Match the coordination compounds in Column I having the given type of hybridisation of $\mathrm{M}^{\mathrm{n}+}$ ion and magnetic moment as given in Column II.
| Coordination compounds | Hybridisation & Magnetic nature | ||
|---|---|---|---|
| A. | $\mathrm{Ni}(\mathrm{CO})_4$ | P. | $\mathrm{sp}^3, \quad \mu=5.92 \mathrm{BM}$ |
| B. | $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ | Q. | $\mathrm{sp}^3, \quad \mu=2.84 \mathrm{BM}$ |
| C. | $\left[\mathrm{Ni}(\mathrm{Cl})_4\right]^{2-}$ | R. | $\mathrm{sp}^3, \quad \mu=0$ |
| D. | $\left[\mathrm{MnBr}_4\right]^{2-}$ | S. | $\mathrm{dsp}^2, \quad \mu=0$ |
An unsaturated hydrocarbon $[\mathrm{A}]$ undergoes the following series of reactions. Identify $[\mathrm{A}]$.

Identify the compound which gives an optically active haloalkane on reaction with $\mathrm{H}_2$ / Ni on heating.
| A | B | C | D |
|---|---|---|---|
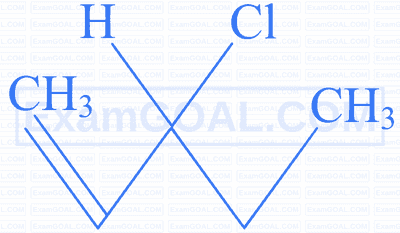 |
 |
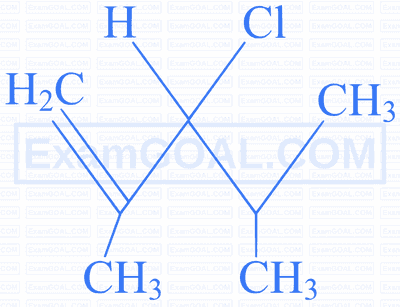 |
 |
Arrange the following in the decreasing order of their $\mathrm{pK}_{\mathrm{a}}$ values.
Cyclohexanol undergoes a series of reactions as given. Identify compound (iv).
$\mathrm{C}_6 \mathrm{H}_{11} \mathrm{OH}+\mathrm{CrO}_3 \rightarrow(\mathrm{i})+\mathrm{C}_6 \mathrm{H}_5 \mathrm{MgI} \rightarrow(\mathrm{ii})+$ dil. $\mathrm{HCl} \rightarrow(\mathrm{iii})+$ Conc. $\mathrm{H}_3 \mathrm{PO}_4 \rightarrow(\mathrm{iv})$
Match the structures in Column I with their correct IUPAC names given in Column II.
| Column I | Column II | ||
|---|---|---|---|
| A. |  |
P. | 2,3-Dibromo-1-phenylpentane. |
| B. | 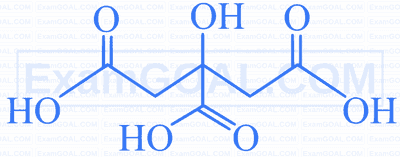 |
Q. | 2,3- Dibromohexanedial. |
| C. | 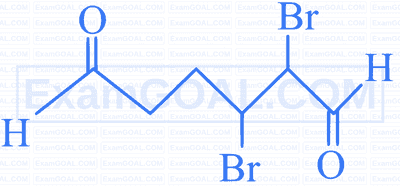 |
R. | 2- ( 4- isobutylphenyl) propanoic acid |
| D. | 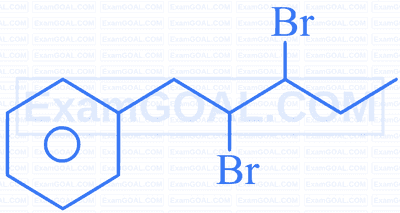 |
S. | 2- Hydroxy-1,2,3- propanetricarboxylic acid. |
Identify $[\mathrm{X}]$, the final product formed when 2 moles of Ethanal undergoes the following series of reactions with reagents [(i) to (iv)]

Two statements, one Assertion (A) and the other Reason (R) are given. Choose the correct option.
Assertion: Maltose, a disaccharide, is a reducing sugar and is obtained by the partial hydrolysis of starch in presence of the enzyme diastase.
Reason: Hydrolysis of one mole of Maltose gives one mole each of $\alpha-D-$ Glucose and $\beta-D-$ Fructose.
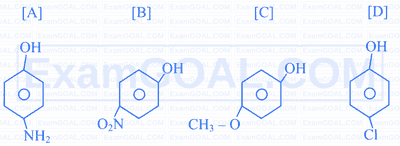
Which one of the following options represents the decreasing order of oxidation number of the central atom in:
$$\mathrm{Cr}_2 \mathrm{O}_7^{2-}, \mathrm{Cr} \mathrm{O}_2^{-}, \mathrm{MnO}_4^{-}, \mathrm{BrO}_3^{-}$$

What is the spin only magnetic moment of the metal ion in $\mathbf{P}$ and the oxidation number of Sulphur in the oxidised product Z ?
Two statements, one Assertion (A) and the other Reason (R) are given. Choose the correct option.
Assertion: 2-aminoethanoic acid and p-aminobenzene sulphonic acid can exist as Zwitter ions while p-aminobenzoic acid cannot.
Reason: When the acid group is a relatively strong proton donor and the $-\mathrm{NH}_2$ group is sufficiently basic it can accept a $\mathrm{H}^{+}$ion from the acid group to form the dipolar ion.
Match the reactions of Glucose given in Column I with the major product formed in the reaction as in Column II.
| Column I - Reactions | Column II - Major product | ||
|---|---|---|---|
| A. | $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{HI}$ (Heat) | P. | $\mathrm{HOOC}-(\mathrm{CHOH})_4-\mathrm{COOH}$ |
| B. | $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{Br}_2(\mathrm{aq})$ | Q. | $\mathrm{CH}_3-\left(\mathrm{CH}_2\right)_4-\mathrm{CH}_3$ |
| C. | $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+$ Conc. $\mathrm{HNO}_3$ | R. | $\mathrm{NH}_4 \mathrm{O}-\mathrm{OC}-(\mathrm{CHOH})_4-\mathrm{CH}_2 \mathrm{OH}$ |
| D. | $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+2\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right] \mathrm{OH}$ | S. | $\mathrm{HOOC}-(\mathrm{CHOH})_4-\mathrm{CH}_2 \mathrm{OH}$ |
The following results were obtained during study of the reaction $2 \mathrm{NO}(\mathrm{g})+\mathrm{Cl}_2(\mathrm{~g}) \rightarrow 2 \mathrm{NOCl}(\mathrm{g})$. Determine the value of $[\mathrm{X}]$ in $\mathrm{mol} / \mathrm{L}$
| Experiment | [NO] mol / L |
[Cl$_2$] mol / L |
Initial rate of formation. [NOCl] mol / L / min |
|---|---|---|---|
| I | 0.2 | 0.2 | $6.0 \times 10^{-3}$ |
| II | 0.2 | 0.4 | $2.4 \times 10^{-2}$ |
| III | 0.4 | 0.2 | $1.2 \times 10^{-2}$ |
| IV | X | 0.6 | $1.35 \times 10^{-1}$ |
Two statements, One Assertion [ A ] and the other Reason [ R ] are given. Identify the correct option
Assertion $[A]$ : The decreasing order of the acidic character of the following is $B>D>A>C$

Reason $[\mathbf{R}]$ : Fluorine has larger -I effect than Cl and Br .
Mathematics
For a given Linear Programming problem, the objective function is
$$z=3 x+2 y$$
Subject to constraints are
$$\begin{aligned} & 4 x+3 y \leq 60 \\ & x \geq 3 \\ & y \leq 2 x \\ & y \geq 0 \end{aligned}$$
P is one of the corner points of the feasible region for the given Linear Programming problem. Then the coordinate of P is
Physics
The nuclear forces
a. are central forces, independent of the spin of the nucleons.
b. have a short-range dominant over a distance of about a few fermi
c. are stronger being hundred times stronger than that of electromagnetic forces.
d. are independent of the nuclear charge.
Which of the above is not true?
For the charge configuration shown here, which of the following is not true?

Which, of the following is true of the Balmer series of the hydrogen spectrum?
a. The series is in the visible region.
b. The entire series falls in the ultraviolet region
c. The entire series falls in the infrared region
d. The series is partly in the visible region and partly in the infrared region
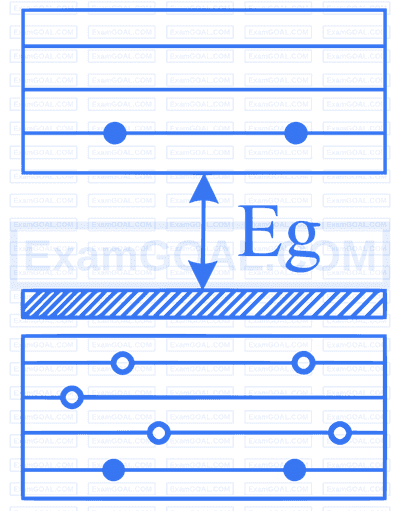
In the energy band diagram of a material shown below, open circles and filled circles denote holes and electrons respectively. The material is a
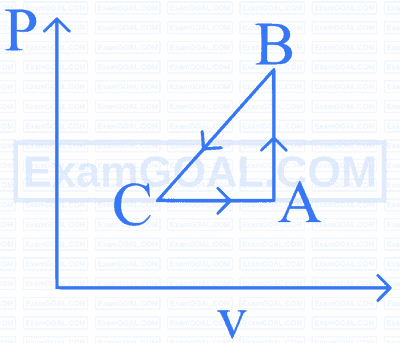
A sample of an ideal gas is taken through the cyclic process ABCA as shown in figure below. It absorbs 60 J of heat during the part AB and rejects 80 J of heat during CA . There is no heat exchanged during the process $\mathrm{BC} . \mathrm{A}$ work of 40 J is done on the gas during the part BC . If the internal energy of the gas at A is 1450 J , then the work done by the gas during the part CA is: