Match the reactions given in Column I with the major product formed given in Column II.
| S.No. | Reactions | S.No. | Major product formed |
|---|---|---|---|
| A. |  |
P. |  |
| B. |  |
Q. | 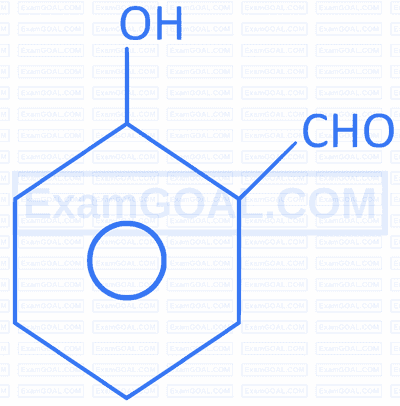 |
| C. | 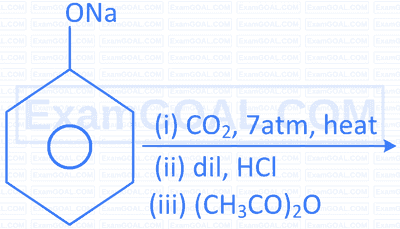 |
R. | 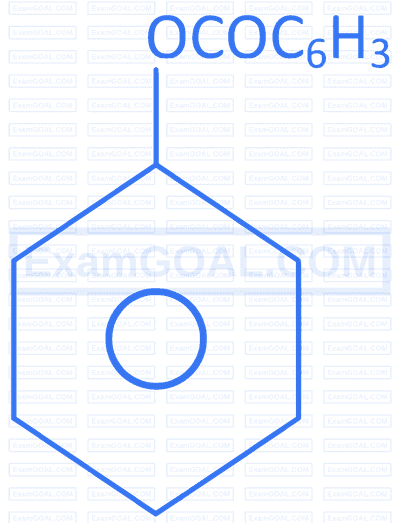 |
| D. | 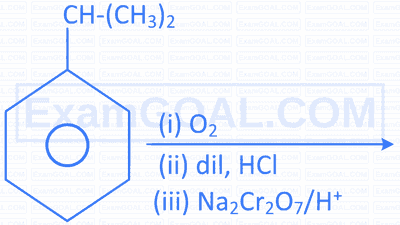 |
S. | 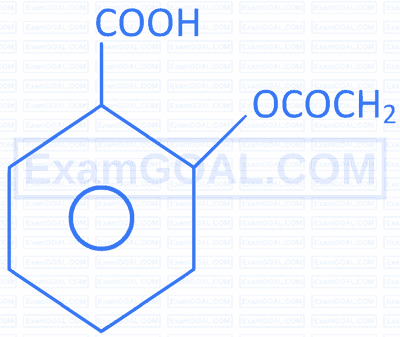 |
Two statements, one Assertion and the other Reason are given. Choose the right option.
Assertion : Insulin is called a protein whereas Glycyl alanine is not called a protein
Reason : A polypeptide with amino acid residue less than 100 can also be called as a protein if it has a well-defined conformation of a protein.
Choose the correct metal/ ion from the brackets which -------------------------
A. has chemical reactivity similar to that of the first few members of the Lanthanoids $(\mathrm{Zn}, \mathrm{Ca}, \mathrm{Fe}, \mathrm{Cu})$.
B. has stable $4 \mathrm{f}^7$ electronic configuration, but acts as a strong reducing agent and converts to $\mathrm{M}^{3+}$ state. $\left(\mathrm{Eu}^{2+}, \mathrm{Ce}^{2+}, \mathrm{Pr}^{2+}, \mathrm{Dy}^{2+}\right)$
C. is a colorless ion $\left(\mathrm{Tm}^{3+}, \mathrm{Lu}^{3+}, \mathrm{Gd}^{3+}, \mathrm{Sm}^{3+}\right)$.
D. shows stable +2 oxidation state and is diamagnetic ( $\mathrm{Ce}, \mathrm{Sm}, \mathrm{Ho}, \mathrm{Yb}$ )