Chemistry
1. An aqueous solution of an electrolyte $\mathrm{A}_3 \mathrm{~B}$ is prepared by dissolving 0.5625 g in 750 ml of water a 2. Arrange the complex ions in the increasing order of their magnetic moments
A. $\left[\mathrm{Fe}\left(\mathrm{H}_2 \math 3. When the temperature of a reaction $\mathrm{A}+\mathrm{B} \rightarrow \mathrm{C}$ is increased from 300 K to 310 K the r 4. The first electron gain enthalpy of oxygen is $-141 \mathrm{~kJ} \mathrm{~mol}^{-1}$, its second electron gain enthalpy 5. Two moles of an ideal gas at 1 bar pressure and 298 K is expanded into vacuum to double the volume. The work done is :
6. The order of a reaction $\mathrm{W}+\mathrm{X} ---- \rightarrow \mathrm{Y}+\mathrm{Z}$ with respect to W is 3 and with r 7. Identify the correct statement.
8. For the cell reaction $4 \mathrm{Br}^{-}+\mathrm{O}_2+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{Br}_2+2 \mathrm{H}_2 \mathr 9. The bond angles in the following molecules decreases in the order. $\mathrm{BF}_3, \mathrm{NH}_3, \mathrm{PF}_3$ and $\m 10. Two statements, one Assertion and the other Reason, are given. Choose the correct option.
Assertion : During the electro 11. An aqueous solution of volume V ml contains a non-volatile solute of unknown mass $W_B \mathrm{~g}$ and molar mass $M_B 12. The ratio of $\mathrm{N}_2$ and $\mathrm{O}_2$ gases in the atmosphere is $4: 1$.
The ratio of the mole fractions of the 13. Identify the correct coefficients (a), (b), (c) and (d) in the following equations
i) $\quad \mathrm{xMnO}_4^{--}+$(a) $ 14. The decomposition of $\mathrm{PH}_3$ follows first order kinetics. The time required for $3 / 4^{\text {th }}$ of $\math 15. An example of disproportionation reaction is :
16. Identify the Alkane (molecular formula $\mathrm{C}_8 \mathrm{H}_{18}$ ) which yields only a single monochloride on Chlor 17. For a reaction,
$$A+B \rightleftharpoons 2 C$$
1.0 mole of $A, 1.5$ mole of $B$ and 0.5 mole of $C$ were taken in a 1 L 18. The correct order of reactivity of halogens with alkanes is :
19. Match the reactions given in Column I with the major product formed given in Column II.
.tg {border-collapse:collapse; 20. The number of grams of bromine that will completely react with 5 g of pentene is :
[Atomic mass of $\mathrm{Br}=80 \math 21. Two statements, one Assertion and the other Reason are given. Choose the right option.
Assertion : Insulin is called a p 22. Choose the correct metal/ ion from the brackets which -------------------------
A. has chemical reactivity similar to th 23. Which of the following alkene on reductive ozonolysis gives ketones only as the product
24. The oxidation number of potassium in $\mathrm{K}_2 \mathrm{O}, \mathrm{K}_2 \mathrm{O}_2$ and $\mathrm{KO}_2$ respective 25. For a cell $2 \mathrm{M}_{(\mathrm{S})}+\mathrm{O}_2(\mathrm{~g})+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{M}^{2+}(\mathrm 26. What would be the major product formed in the reaction?
27. When $0.1 \mathrm{~mol} \mathrm{~L}^{-1}$ of KCl was filled in a Conductivity cell the resistance was 80 ohms at 298 K . 28. An optically active compound $[\mathrm{X}]$ (Molecular formula $\mathrm{C}_8 \mathrm{H}_{11} \mathrm{~N}$ ) reacts with 29. Choose the correct statement from the options given
30. The number of electrons with azimuthal quantum numbers $l=1$ and $l=2$ for Cr in the ground state electronic configurati 31. The ratio of the difference between the radii of $3^{\text {rd }}$ and $4^{\text {th }}$ orbits of the $\mathrm{He}^{+}$ 32. Consider the reaction
$$\mathrm{Fe}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{CO}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{ 33. The electronic configuration of the element with atomic number 78 is :
34. Identify the final product $[\mathrm{Y}]$ in the following reaction.
35. Choose the incorrect statement. 36. Find the correct matches.
.tg {border-collapse:collapse;border-spacing:0;}
.tg td{border-color:black;border-style:soli 37. Which of the following compounds has electrons symmetrically distributed in both $\mathrm{t}_{2 \mathrm{~g}}$ and $\math 38. Identify the two incorrect electrochemical reactions shown as taking place in the given cells at the respective electrod 39. Choose the correct statement.
40. Consider the following reaction
$$\text { Propyne }+\mathrm{CH}_3 \mathrm{MgBr} \xrightarrow{\text { dry ether }} \mathb 41. Match the IUPAC names in Column II with the correct structures given in Column I.
.tg {border-collapse:collapse;border 42. An organic compound $[\mathrm{X}]$ (Molecular formula $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_2$ ) reacts with dil. $\m 43. In the decomposition of limestone to lime, the values of $\Delta H^o$ and $\Delta S^o$ are $+179.1 \mathrm{~kJ} \mathrm{ 44. The mole fraction of an unknown solute in 1560 g of Benzene is 0.5 . What is the molality of the solution? (M. M of Benz 45. Which of the coordination compounds [(i) to (v)] are used for the following processes :
A: Electroplating
B: Removal of 46. Identify the type of reaction:
47. Identify X, Y and Z formed in the reaction:
48. Which of the following is a water soluble vitamin?
49. At 300 K the vapour pressure of an ideal solution containing 1.0 mole each of volatile liquids X and Y is 1000 mm . Keep 50. The maximum polarity and dipole moment among the following is : 51. During a chemical reaction $\mathrm{X} \rightarrow \mathrm{Y}$, the rates of reaction starting with initial concentratio 52. Identify the Carbonyl compound which will not be formed when hydration of Alkynes is carried out with dil. $\mathrm{H}_2 53. Choose the incorrect statement from the following.
54. Identify $[\mathrm{X}]$ used in the given reaction.
$[\mathrm{X}]+$ Copper $/ 573 \mathrm{~K} \rightarrow[\mathrm{Y}]$
$ 55. Which one of the following is the product $(\mathrm{Z})$ formed at the end of the given reaction:
$$\begin{aligned}
& \t 56. Two statements, one Assertion and the other Reason are given. Identify the correct option
Assertion : Primary and second 57. The enthalpies of combustion of $\mathrm{H}_2, \mathrm{C}$ (graphite) and $\mathrm{C}_2 \mathrm{H}_6(\mathrm{~g})$ are $ 58. Which of the following is the most stable free radical?
59. What are the intermediates formed during the reactions $A$ and $B$ ?
A. Reimer-Tiemann reaction.
B. Dehydration of alcoh 60. Arrange the following in the increasing order of their covalent character.
$\mathrm{CaF}_2 ; \mathrm{CaCl}_2 ; \mathrm{C
Mathematics
1. A function $f$ from the set of natural numbers to integers defined by
$$f(n)=\left\{\begin{array}{l}
\frac{n-1}{2}, \qua 2. The value of $\frac{\sin ^2 20^{\circ}+\cos ^4 20^{\circ}}{\sin ^4 20^{\circ}+\cos ^2 20^{\circ}}$ is : 3. $$\int \frac{\sin 2 x}{(1+\sin x)(2+\sin x)} d x=a \log |1+\sin x|-b \log |2+\sin x|+c$$
then the value of $a$ and $b$ 4. The solution for the following system of inequalities $3 x-7 5. Kiran purchased 3 pencils, 2 notebooks and one pen for ₹41.
From the same shop Manasa purchased 2 pencils, one notebook 6. The area of a triangle formed by the lines joining the vertex of the parabola $x^2=\lambda y$ to the ends of its latus r 7. If $X=\left[\begin{array}{ll}1 & 0 \\ 0 & 1\end{array}\right]$ and $Y=\left[\begin{array}{cc}0 & 1 \\ -1 & 0\end{array}\ 8. Two numbers are selected at random from integers 1 to 9 .
If their sum is even, what is the probability that both the nu 9. If the standard deviation of $0,1,2,3 \cdots\cdots\cdots9$ is ' $k$ ' then the standard deviation of $10,11,12,13, \cdot 10. If $A=\left[\begin{array}{ll}a & b \\ b & a\end{array}\right]$ and $(A I)^2=\left[\begin{array}{ll}\alpha & \beta \\ \be 11. Let $f(x)=x \sqrt{4 a x-x^2}, a>0$ then $f^{\prime}(x)$ at $x=2 a$ is : 12. The angle between two lines is $45^{\circ}$ and slope of one line is $\frac{1}{4}$ then which is the possible value of t 13. Given $Z=80 x+120 y$, subject to constraints are $x+3 y \leq 30 ; 3 x+4 y \leq 60 ; x \geq 0 ; y \geq 0$.
P is one of th 14. If $\sin x+\sin ^2 x=1$ then $\cos ^8 x+2 \cos ^6 x+\cos ^4 x$ is equal to : 15. $\int_0^1 x(1-x)^{99} d x=$ 16. Value of the determinant of a matrix $A$ of order $3 \times 3$ is 7 . Then the value of the determinant formed by the co 17. The relationship between a and b for the continuous function
$f(x)=\left\{\begin{array}{ll}a x+1, & \text { if } x \leq 18. If the function $f(x)=\mu \sin x+\frac{1}{3} \sin 3 x$ has its derivative equal to zero at $x=\frac{\pi}{3}$, then the v 19. A man is moving away from a tower 41.6 m high at a rate of $2 \mathrm{~m} / \mathrm{s}$. If the eyelevel of the man is 1 20. $\sin ^{-1}(x-1)+\cos ^{-1}(x-3)+\tan ^{-1}\left(\frac{x}{2-x^2}\right)=\cos ^{-1} k+\pi$, then the value of ' $k$ ' is
21. Given that $z$ is a real number and $z=\frac{\lambda+4 i}{1+\lambda i}$ where $\lambda \in R$, then the possible value o 22. If $z=\left(\frac{\sqrt{3}}{2}+\frac{i}{2}\right)^5+\left(\frac{\sqrt{3}}{2}-\frac{i}{2}\right)^5$, then
23. The solution of the differential equation: $x \cos y d y=\left(x e^x \log x+e^x\right) d x$ is
24. Area of the region bounded by the curve $y=\cos x$ between $x=-\frac{\pi}{2}$ and $x=\pi$ is ------------------
25. If a quadratic function in $x$ has the value 19 when $x=1$ and has a maximum value 20 when $x=2$, then the function is
26. If A and B are two events such that $P(\bar{A})=0.3, P(B)=0.4, P(A \cap \bar{B})=0.5$, then find the value of $P(B / A \ 27. If $\lim\limits_{x \rightarrow 1} \frac{x^4-1}{x-1}=\lim\limits_{x \rightarrow k} \frac{x^3-k^3}{x^2-k^2}$, then the val 28. $\int\left(e^{x \log _e 6}\right) e^x d x=\phi(x)+c$ then $\phi(x)=$ 29. The number of solutions of $\frac{d y}{d x}=\frac{y+1}{x-1}$, when $y(1)=2$ is : 30. Evaluate: $\lim _\limits{x \rightarrow 0} \frac{\sqrt[3]{1+x}-\sqrt[3]{1-x}}{x}$ 31. A geometric progression consists of an even number of terms. If the sum of all the terms is five times the sum of the te 32. Find the function ' $f$ ' which satisfies the equation $\frac{d f}{d x}=2 f$, given that $f(0)=e^3$
33. In a game, a man wins ₹ 1000 if he gets an even number greater than or equal to 4 on a fair dice and loses ₹ 200 for get 34. The number of words that can be formed with the letters of the word 'DEFINITE' if two vowels are together and the other 35. The relation $R=\{(1,1),(2,2),(3,3)\}$ on the set $\{1,2,3\}$ is
36. If the length of the diagonal of a square is increasing at the rate of $0.1 \mathrm{~cm} / \mathrm{sec}$.
What is the r 37. Two finite sets have $m$ and $n$ elements. The total number of proper subsets of the first set is 119 more than the tota 38. Given that n number of arithmetic means are inserted between two pairs of numbers $a, 2 b$ and $2 a, b$; where $a, b \in 39. $\int\limits_{-2}^2 \frac{|x-3|}{x-3} d x=$ 40. If $x=a\left[\left\{\cos t+\frac{1}{2} \log \left(\tan ^2 \frac{t}{2}\right)\right\}\right]$ and $y=a \sin t$ then $\fra 41. If the area under the curve $y=\sqrt{a^2-x^2}$ included between the lines $x=0$ and $x=a$ is 4 sq units. Then the value 42. The function $y=\frac{\log x}{x^3}$ is strictly increasing function for
43. If $P=\{5 m: m \in N\}$ and $Q=\left\{5^m: m \in N\right\}$, where $N$ is set of natural numbers, then
44.
The image of a point $P(3,5,3)$ in the line $\frac{x}{1}=\frac{y-1}{2}=\frac{z-2}{3}$ is $P^{\prime}(a, b, c)$. Then $a 45. If $\vec{a}$ and $\vec{b}$ are two vectors such that $\vec{a} \cdot \vec{b}=|\vec{a} \times \vec{b}|$ then the angle bet 46. How many natural numbers are there between 100 and 1000 such that at least one of their digits is $6 ?$
47. For any vector $\vec{p}$, the value of $\left[2\left\{|\vec{p} \times \hat{\imath}|^2+|\vec{p} \times \hat{\jmath}|^2+|\ 48. $\int_{\frac{\pi}{6}}^{\frac{\pi}{3}} \frac{1}{1+\sqrt{\tan x}} d x=$ 49. A pot contains 5 red and 2 green balls. A ball is drawn at random from this pot. If a drawn ball is green, then a red ba 50. If $A=\left[\begin{array}{ccc}4 & \lambda & -3 \\ 0 & 2 & 5 \\ 1 & 1 & 3\end{array}\right]$ then $A^{-1}$ exists if :
51. The value of $\tan \left\{\cos ^{-1}\left(\frac{\sqrt{2}}{2}\right)-\frac{\pi}{2}\right\}$ is
52. If the line $(3 x+14 y+7)+k(5 x+7 y+6)=0$ is perpendicular to $x$-axis then the value of ' $k$ ' is
53. A straight line makes positive intercepts on the coordinate axes whose sum is 5 . If the line passes through the point $ 54. If the foci of the ellipse $\frac{x^2}{16}+\frac{y^2}{b^2}=1$ and the foci of the hyperbola $\frac{x^2}{144}-\frac{y^2}{ 55. The equation of a line passing through origin with direction angles $\frac{2 \pi}{3}, \frac{\pi}{4}, \frac{\pi}{3}$ is
56. Two lines $\frac{x-1}{2}=\frac{y+1}{3}=\frac{z-1}{4}$ and $\frac{x-3}{1}=\frac{y-k}{2}=\frac{z}{1}$ intersect at a point 57. Solve the following differential equation $\cos ^2 x \frac{d y}{d x}+y=\tan x$, given that $y(0)=1$. Hence find $y\left( 58. If $\tan \alpha=\frac{1}{7}$ and $\sin \beta=\frac{1}{\sqrt{10}}, \quad 0 59. An unbiased die is tossed twice. What is the probability of getting a 4,5 or 6 on the first toss and a $1,2,3$ or 4 on t 60. If $y=x+e^x$ then $\frac{d^2 x}{d y^2}=$
Physics
1. The interference pattern is obtained with two coherent light sources of intensity ratio $9: 1$.
The ratio of $\frac{I_{\ 2. If the mass numbers of two nuclei are in the ratio $5: 2$ and their diameters are in ratio $2: 6$. Then their nuclear de 3. Which of the following is correct in the case of the Bohr model of atoms?
A. Predicts continuous emission spectra for al 4. The zener voltage in the circuit shown is $\mathrm{V}_{\mathrm{Z}}=20 \mathrm{~V}$. The load resistance $\mathrm{R}_{\ma 5. The unit of universal gravitational constant is :
6. In the normal adjustment of an astronomical telescope, the objective and eyepiece are 36 cm apart. If the magnifying pow 7. A rectangular coil of 250 turns has an average area of $20 \mathrm{~cm} \times 15 \mathrm{~cm}$. The coil rotates with a 8. A beam of incident parallel light falls on a diverging lens of focal length 20 cm in magnitude. If a converging lens of 9. The total number of degrees of freedom associated with $2 \mathrm{~cm}^3$ of Nitrogen gas at normal temperature and pres 10. A bob of a simple pendulum has a mass of 4 g and a charge of $20 \mu \mathrm{C}$. If it is at rest in a uniform horizont 11. Three charges, $Q,-q$ and $2 q$ are placed at the vertices of a right-angled isosceles triangle. What is the value of $q 12. The minimum energy required by a hydrogen atom in ground state to emit radiation in Paschen series is nearly: 13. An object of height $h$ is placed midway between $f$ and $2 f$ in front of a biconvex lens. A real inverted image is cap 14. A power transmission line feeds input power at 2200 V to a step-down transformer with its primary windings having 2000 t 15. Two physical quantities having the same dimensional formula $\left[\mathrm{M}^1 \mathrm{~L}^{-1} \mathrm{~T}^{-2}\right] 16. The stopping potential when a metal surface is illuminated by light of wavelength $\lambda$ is 15 V . The stopping poten 17. A wire of negligible mass having uniform area of cross section ' A ' and young modulus ' Y ' is used to suspend a point 18. Applying a constant torque the speed of a flywheel is increased from 1800 rpm to 2400 rpm in 10 seconds. The number of r 19. A short bar magnet placed with its axis at $45^{\circ}$ with an external field of $400 \times 10^{-4} \mathrm{~T}$ exper 20. If the distance between the Sun and Earth is doubled, then the duration of the year on earth will be :
[Given actual dur 21. The rms velocity of the gas molecule at $327^{\circ} \mathrm{C}$ is same as the rms velocity of the oxygen molecules at 22. Select the correct statement from the following: The position of the centre of mass of a system :
23. A particle of mass 3 g and charge $60 \mu \mathrm{C}$ is released from rest in a uniform electric field of intensity $10 24. A network of capacitors is as shown below. If the voltage supply is 100 V , find the energy stored in the $6 \mu \mathrm 25. A body of mass 10 kg is moving up on an inclined plane of $30^{\circ}$ with an acceleration $2 \mathrm{~ms}^{-2}$. Find 26. A galvanometer having a resistance of $50 \Omega$ is shunted by a wire of resistance $10 \Omega$. If the total current i 27. The power dissipated across the $16 \Omega$ resistor in the circuit is 2 watts. The power dissipated in watt units acros 28. Select the graph which represents the motion of a particle along a straight line with uniform acceleration. 29. A metal rod of susceptibility 799 is subjected to a magnetising field of $2000 \mathrm{Am}^{-1}$. The permeability of th 30. A given volume of gas at NTP is allowed to expand 6 times of its original volume, first under isothermal condition and t 31. The net resistance between the points A and B in the circuit given below is:
32. The wavelength of a monochromatic light which is used in single slit diffraction is 800 nm . The width of the single sli 33. A plane electromagnetic wave with frequency 40 MHz travels in free space. At a particular point in space and time, the m 34. A series LCR circuit having $\mathrm{R}=44 \Omega, \mathrm{~L}=2 \mathrm{H}$ and $\mathrm{C}=25 \mu \mathrm{~F}$ is conn 35. Momentum of a body is increased to three times its original value. By what percentage will its kinetic energy change?
36. What is the dc component of the output voltage if a sinusoidal signal of 33 V peak voltage is the input of a half wave d 37. Radium having mass number 200 and binding energy per nucleon 5.6 MeV , splits into two fragments Cadmium of mass number 38. In a particular case X , a certain length of insulated copper wire is bent to form double loops of equal radii. In anoth 39. The fundamental frequency of sound produced in an open pipe of length $\mathbf{L}_1$ is same as the frequency of the $3^ 40. Select the correct statement from the following:
41. A straight wire of mass 250 g and length 2.5 m carries a current of 4 A . It can be suspended in mid air by a uniform ho 42. Select the correct statement from the following:
43. A boy is running along a straight horizontal road with a constant speed $5 \mathrm{~ms}^{-1}$. While running he throws a 44. In a pure inductive circuit, a sinusoidal voltage $V(t)=200 \sin 250 t$ is applied to a pure inductance of $\mathrm{L}=0 45. In to a vessel containing pure water a clean glass tube of radius $3.6 \times 10^{-4} \mathrm{~m}$ is held vertically wi 46. A point marked on a ring of radius 2 cm is in contact with a horizontal plane. Now the ring is rolled forward half a rev 47. The instantaneous values of alternating current and voltages in a circuit are $\mathrm{I}=\frac{3}{\sqrt{2}} \sin (200 \ 48. The velocity - mass graph of body with constant linear momentum is represented by the graph: 49. A student measures the terminal potential difference $V$ of a cell of emf $\varepsilon$ and internal resistance $r$ as a 50. Young's double slit experiment is first done in air and then in a medium of refractive index $\mu$. If the 7 th dark fri 51. When ${ }^{10} \mathrm{~B}_5$ nuclei are bombarded by neutrons, one of the resultant nuclei is ${ }^7 \mathrm{Li}_3$. Th 52. The resistance of a heating element is found to be $120 \Omega$ at room temperature which is $20^{\circ} \mathrm{C}$. If 53. A plot of kinetic energy of emitted photoelectrons from a metal versus the frequency of incident radiation gives a strai 54. A plano-convex lens made of refractive index 1.5 and having radius of curvature $\mathrm{R}=4 \mathrm{~cm}$ fits exactly 55. The rate of heat conduction in the given two metal rods having the same length is found to be the same when the temperat 56. A current carrying closed loop in the form of a right isosceles triangle XYZ is placed in a uniform magnetic field $B$ a 57. A capacitor of capacitance $4 \mu \mathrm{~F}$ is charged to a potential of 24 V and then connected in parallel to an un 58. A uniformly charged conducting sphere of 0.2 m diameter has a surface charge density of $70 \mu \mathrm{Cm}^{-2}$. The e 59. An intrinsic semiconductor has equal concentrations of hole and electron which is equal to $4 \times 10^8 \mathrm{~m}^{- 60. Two electric dipoles of dipole moments $3.9 \times 10^{-30} \mathrm{Cm}$ and $5.2 \times 10^{-30} \mathrm{Cm}$ are place
1
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
Which of the following is a water soluble vitamin?
A
Vitamin C
B
Vitamin E
C
Vitamin A
D
Vitamin D
2
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
At 300 K the vapour pressure of an ideal solution containing 1.0 mole each of volatile liquids X and Y is 1000 mm . Keeping the temperature constant, when 2.0 moles of liquid X is added to the solution, its vapour pressure increases by 200 mm . Calculate the vapour pressure of X and Y in their pure state
A
$P_X^0=1400 \quad P_Y^0=600$
B
$P_X^0=1700 \quad P_Y^0=400$
C
$P_X^0=1200 \quad P_Y^0=480$
D
$P_X^0=1000 \quad P_Y^0=500$
3
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
The maximum polarity and dipole moment among the following is :
A
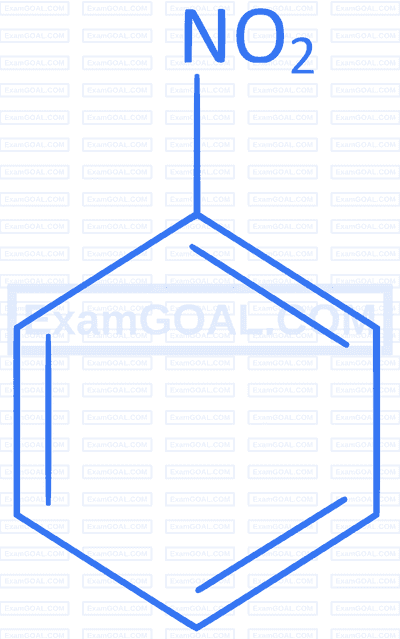
B
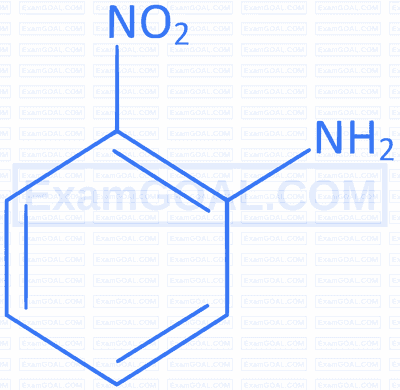
C
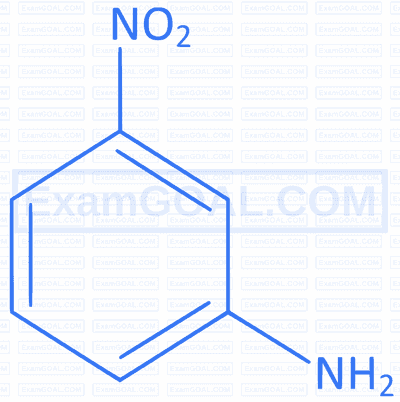
D

4
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
During a chemical reaction $\mathrm{X} \rightarrow \mathrm{Y}$, the rates of reaction starting with initial concentrations of X as $4.0 \times 10^{-3} \mathrm{M}$ and $2.0 \times 10^{-3} \mathrm{M}$ are $4.8 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} / \mathrm{s}$ and $1.2 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} / \mathrm{s}$ respectively. What is the order of reaction with respect to X ?
A
2
B
3
C
1.5
D
1
Paper analysis
Total Questions
Chemistry
60
Mathematics
60
Physics
60
COMEDK
Papers
2025
2024
2022
2021
2020