
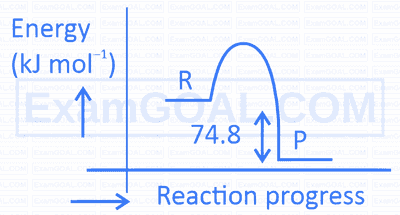
$\mathrm{C}(\mathrm{s})+2 \mathrm{H}_2(\mathrm{~g}) \rightarrow \mathrm{CH}_4(\mathrm{~g}) ; \Delta \mathrm{H}=-74.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$. Which of the following diagrams gives an accurate representation of the above reaction? [ $\mathrm{R} \rightarrow$ reactants; $\mathrm{P} \rightarrow$ products]




Sugar ' $X$ '
A. is found in honey
B. is a keto sugar
C. exists in $\alpha$ and $\beta$-anomeric forms.
D. Is laevorotatory.
' X ' is :

Total number of possible isomers (both structural as well as stereoisomers) of cyclic ethers of molecular formula $\mathrm{C}_4 \mathrm{H}_8 \mathrm{O}$ is :

For the reaction $\mathrm{A}(\mathrm{g}) \rightleftharpoons 2 \mathrm{~B}(\mathrm{~g})$, the backward reaction rate constant is higher than the forward reaction rate constant by a factor of 2500 , at 1000 K.
[Given : $\mathrm{R}=0.0831 \mathrm{~L} \mathrm{~atm} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$ ]
$K_p$ for the reaction at $1000 K$ is