NEET 2016 Phase 2
Paper was held on
Sun, Jul 24, 2016 10:00 AM
Biology
1
Choose the correct statement.
2
Which of the following biomolecules is common to respiration-mediated breakdown of fats, carbohydrates and proteins?
3
You are given a tissue with its potential for differentiation in an artificial culture. Which of the following pairs of hormones would you add to the medium to secure shoots as well as roots?
4
Phytochrome is a
5
The partial pressure of oxygen in the alveoli of the lungs is
6
Lungs do not collapse between breaths and some air always remains in the lungs which can never be expelled because
7
Name the blood cells, whose reduction in number can cause clotting disorder, leading to excessive loss of blood from the body.
8
Serum differs from blood in
9
The part of nephron involved in active reabsorption of sodium is
10
Name the ion responsible for unmasking of active sites for myosin for cross-bridge activity during muscle contraction.
11
Osteoporosis, an age-related disease of skeletal system, may occur due to
12
Oxidative phosphorylation is
13
Graves' disease is caused due to
14
Name a peptide hormone which acts mainly on hepatocytes, adipocytes and enhances cellular glucose uptake and utilisation.
15
The posterior pituitary gland is not a 'true' endocrine gland because
16
Pollination in water hyacinth and water lily is brought about by the agency of
17
In majority of angiosperms
18
The ovule of an angiosperm is technically equivalent to
19
Match column I with column II and select the correct option using the codes given below.
| Column I | Column II | ||
|---|---|---|---|
| (A) | Mons pubis | (i) | Embryo formation |
| (B) | Antrum | (ii) | Sperm |
| (C) | Trophectoderm | (iii) | Female external genitalia |
| (D) | Nebenkern | (iv) | Graafian follicle |
20
How many plants among Indigofera., Sesbania, Salvia, Allium, Aloe, mustard, groundnut, radish, gram and turnip have stamens with different lengths in their flowers?
21
Match column I with column II for housefly classification and select the correct option using the codes given below.
| Column $${\rm I}$$ | Column $${\rm I}$$$${\rm I}$$ | |||
|---|---|---|---|---|
| (A) | Family | (i) | Diptera | |
| (B) | Order | (ii) | Arthropoda | |
| (C) | Class | (iii) | Muscidae | |
| (D) | Phylum | (iv) | Insecta | |
22
Study the four statements (A-D) given below and select the two correct ones out of them.
A. Definition of biological species was given by Ernst Mayr.
B. Photoperiod does not affect reproduction in plants.
C. Binomial nomenclature system was given by R.H. Whittaker.
D. In unicellular organisms, reproduction is synonymous with growth.
The two correct statements are
A. Definition of biological species was given by Ernst Mayr.
B. Photoperiod does not affect reproduction in plants.
C. Binomial nomenclature system was given by R.H. Whittaker.
D. In unicellular organisms, reproduction is synonymous with growth.
The two correct statements are
23
Which one of the following is wrong for fungi?
24
Select the wrong statement.
25
Methanogens belong to
26
Conifers are adapted to tolerate extreme environmental conditions because of
27
Which one of the following statements is wrong?
28
Choose the correct statement
29
The term 'polyadelphous' is related to
30
Free-central placentation is found in
31
Which of the following depicts the correct pathway of transport of sperms?
32
Radial symmetry is found in the flowers of
33
Which of the following is the least likely to be involved in stablishing the three-dimensional folding of most proteins?
34
A non-proteinaceous enzyme is
35
Which of the following describes the given graph correctly?


36
During cell growth, DNA synthesis takes place on
37
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated?
38
Match the stages of meiosis in column I to their characteristic features in column II and select the correct option using the codes given below.
| Column $${\rm I}$$ | Column $${\rm I}$$$${\rm I}$$ | ||
|---|---|---|---|
| A. | Pachytene | (i) | Pairing of homologous chromosomes |
| B. | Metaphase $${\rm I}$$ | (ii) | Terminalisation of chiasmata |
| C. | Diakinesis | (iii) | Crossing-over takes place |
| D. | Zygotene | (iv) | Chromosomes align at equatorial plate |
39
The process which makes major difference between C3 and C4 plants is
40
Which of the following National Parks is home to the famous musk deer or hangul ?
41
A foreign DNA and plasmid cut by the same restriction endonuclease can be joined to form a recombinant plasmid using
42
Which of the following is not a component of downstream processing ?
43
Which of the following restriction enzymes produces blunt ends ?
44
Which kind of therapy was given in 1990 to a four-year-old girl with adenosine deaminase [ADA] deficiency ?
45
If '+' sign is assigned to beneficial interaction, '–' sign to detrimental and '0' sign to neutral interaction, then the population interaction represented by '+' '–' refers to
46
Which of the following is correct for r-selected species ?
47
The principle of competitive exclusion was stated by
48
The primary producers of the deep-sea hydrothermal vent ecosystem are
49
How many hot spots of biodiversity in the world have been identified till date by Norman Myers ?
50
Which of the following is correctly matched ?
51
Red List contains data or information on
52
Stirred-tank bioreactors have been designed for -
53
The balloon-shaped structures called tyloses
54
Cortex is the region found between -
55
In male cockroaches, sperms are stored in which part of the reproductive system ?
56
Smooth muscles are
57
A cell organelle containing hydrolytic enzymes is
58
Select the mismatch.
59
Select the wrong statement-
60
DNA-dependent RNA polymerase catalyzes transcription on one strand of the DNA which is called the
61
Several hormones like hCG, hPL, estrogen, progesterone are produced by
62
Which of the following is hormone releasing IUD ?
63
Which of the following is incorrect regarding vasectomy ?
64
Embryo with more than 16 blastgomeres formed due to in vitro fertilization is transferred into
65
The mechanism that causes a gene to move from one linkage group to another is called
66
If a colour-blind man marries a woman who is homozygous for normal colour vision, the probability of their
son being colour-blind is
67
Taylor conducted the experiments to prove semiconservative mode of chromosome replication on
68
The equivalent of a structural gene is -
69
A molecule that can act as a genetic material must fulfill the traits given below, except
70
Which of the following rRNAs acts as structural RNA as well as ribozyme in bacteria ?
71
The label of a herbarium sheet does not carry information on
72
Genetic drift operates in
73
In Hardy-Weinberg equation, the frequency of heterozygous individual is represented by
74
The chronological order of human evolution from early to the recent is
75
Which of the following is the correct sequence of events in the origin of life ?
I. Formation of protobionts
II. Synthesis of organic monomers
III. Synthesis of organic polymers
IV. Formation of DNA-based genetic systems
I. Formation of protobionts
II. Synthesis of organic monomers
III. Synthesis of organic polymers
IV. Formation of DNA-based genetic systems
76
Which of the following is correct regarding AIDS causative agent HIV ?
77
Which of the following sets of diseases is caused by bacteria ?
78
Match Column-I with Column-II and select the correct option using the codes given below –
| Column I | Column II |
|---|---|
| (a) Pistils fused together | (i) Gametogenesis |
| (b) Formation of gametes | (ii) Pistillate |
| (c) Hyphae of higher Ascomycetes | (iii) Syncarpous |
| (d) Unisexual female flower | (iv) Dikaryotic |
Chemistry
1
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weights 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
2
If the Eocell for a given reaction has a negative value, which of the following gives the correct relationships for the values of $$\Delta $$Go and Keq ?
3
The correct structure of the product 'A' formed in the reaction


4
The correct order of strengths of the carboxylic acids

is

is
5
Which one of the following nitro-compounds does not react with nitrous acid?
6
A given nitrogen-containing aromatic compound 'A' reacts with Sn/HCl, followed by HNO2 to give instable compound 'B'. 'B', on treatment with phenol, forms a beautiful coloured compound 'C' with the molecular formula C12H10N2O. The structure of compound 'A' is
7
The central dogma of molecular genetics states that the genetic information flows from
8
The correct corresponding order of names of four aldoses with configuration given below

respectively, is

respectively, is
9
The van't Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
10
Which one of the following is incorrect for ideal solution?
11
The molar conductivity of a 0.5 mol/dm3 solution of AgNO3 with electrolytic conductivity of 5.76 $$ \times $$ 10$$-$$3 S cm$$-$$1 at 298 K is
12
During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
13
Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
14
The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (charge on electron = 1.60 $$ \times $$ 10$$-$$19C)
15
The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
16
Boric acid is an acid because its molecule
17
AlF3 is soluble in HF only in presence of KF. It is due to the formation of
18
Which one of the following statements related to lanthanoids is incorrect?
19
Jahn-Teller effect is not observed in high spin complexes of
20
The correct increasing order of trans-effect of the following species is
21
The solubility of AgCl(s) with solubility product 1.6 $$ \times $$ 10$$-$$10 in 0.1 M NaCl solution would be
22
How many electrons can fit in the orbital for which n = 3 and $$l$$ = 1?
23
Which of the following pairs of d-orbitals will have electron density along the axes ?
24
Which one of the following compounds shows the presence of intramolecular hydrogen bond?
25
Which of the following pairs of ions is isoelectronic and isostructural ?
26
The hybridizations of atomic orbitals of nitrogen in NO$$_2^ + $$, NO$$_3^ - $$ and NH$$_4^ + $$ respectively are
27
The correct geometry and hybridization for XeF4 are
28
Among the following, which one is a wrong statement ?
29
For a sample of perfect gas when its pressure is changed isothermally from pi to pf, the entropy change is given by
30
The percentage of pyridine (C5H5N) that forms pyridinium ion (C5H5N+H) ina 0.10 M aqueous pyridine solution (Kb for C5H5N = 1.7 $$ \times $$ 10$$-$$9) is
31
Which of the following fluro-compounds is most likely to behave as a Lewis base ?
32
Which among the given molecules can exhibit tautomerism?


33
Which of the following can be used as the halide component for Friedel-Crafts reaction?
34
In which of the following molecules, all atoms are coplanar?
35
In pyrrole the electron density is maximum on


36
In the given reaction,

the product P is

the product P is
37
Which of the following compounds shall not produce propene by reaction with HBr followed by elimination or direct only elimination reaction ?
38
The compound that will react most readily with gaseous bromine has the formula
39
Consider the reaction,
CH3CH2CH2Br + NaCN $$ \to $$ CH3CH2CH2CN + NaBr
This reaction will be the fastest in
CH3CH2CH2Br + NaCN $$ \to $$ CH3CH2CH2CN + NaBr
This reaction will be the fastest in
40
Hot concentrated sulphate acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behaviour?
Physics
1
A person can see clearly objects only when they lie between 50 cm and 400 cm from his eyes . In order to increase the maximum distance of distinct vision to infinity, the type and power of the correcting lens, the person has to use. will be
2
A parallel-plate capacitor of area a, plate separation d and capacitance C is filled with four dielectric materials having dielectric constants k1, k2, k3 and k4 as shown in the figure. If a single dielectric material is to be used to have the same capacitance C in this capacitor, then its dielectric constant k is given by


3
The potential difference (VA $$-$$ VB) between the points A and B in the given figure is
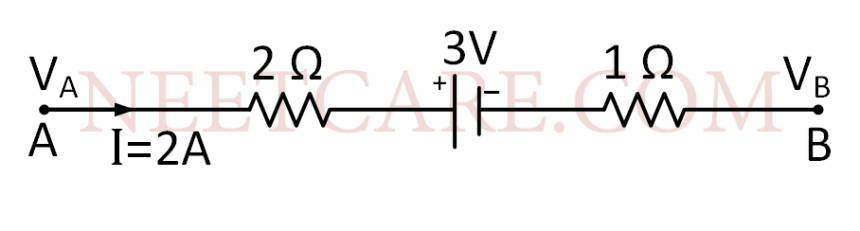
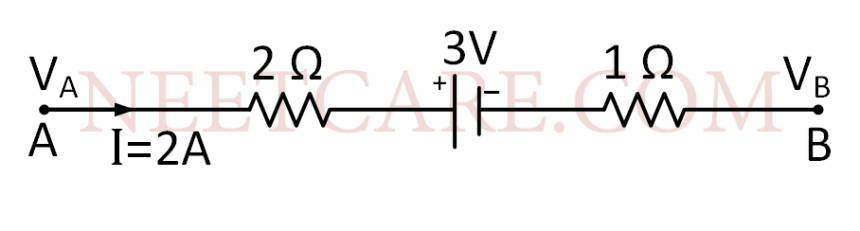
4
A filament bulb (500 W, 100 V) is to be used in a 230 V main supply. When a resistance R is connected in series, it works perfectly and the bulb consumes 500 W. The value of R is
5
A long wire carrying a steady current is bent into a circular loop of one turn. The magnetic field at the centre of the loop is B. It is then bent into a circular coil of n turns. The magnetic field at the centre of this coil of n turns will be
6
An electron is moving in a circular path under the influence of a transverse magnetic field of 3.57 $$ \times $$ 10-2 T. If the value of e/m is 1.76 $$ \times $$ 1011 C kg$$-$$1, the frequency of revoluation of the electron is
7
A bar magnet is hung by a thin cotton thread in a uniform horizontal magnetic field and is in equilibrium state. The energy required to rotate it by 60o is W. Now the torque required to keep the magnet in this new position is
8
Which of the following combinations should be selected for better tuning of an L-C-R circuit used for combination ?
9
A uniform magnetic field is restricted within a region of rafius r. The magnetic field changes with time at a rate $${{d\overrightarrow B } \over {dt}}$$. Loop 1 of radius R > r encloses the region r and loop 2 of radius R is outside the region of magnetic field as shown in the figure. Then the e.m.f. generated is


10
The potential differences across the resistance, capacitance and inductance are 80 V, 40 V and 100 V respectively in an L-C-R circuit. The power factor of this circuit is
11
A 100 $$\Omega $$ resistance and a capacitor of 100 $$\Omega $$ reactance are connected in series across a 220 V source. When the capacitor is 50% charged, the peak value of the displacement current is
12
Two identical glass ($$\mu $$g = 3/2) equiconvex lenses of focal length $$f$$ each are kept in contact. The space between the two lenses is filled with water $$\left( {{\mu _w} = 4/3} \right)$$. The focal length of the combination is
13
An electric dipole is placed at an angle of 30o with an electric field intensity 2 $$ \times $$ 105 N C$$-$$1. It experiences a torque equal to 4 N m. The charge on the dipole, if the dipole length is 2 cm, is
14
An air bubble in a glass slab with refractive index 1.5 (near normal incidence) is 5 cm deep when viewed from one surface and 3 cm deep when viewed from the opposite face. The thickness (in cm) of the slab is
15
The interference pattern is obtained with two coherent light sources of intensity ratio n. In the interference pattern, the ratio $${{{I_{max}} - {I_{\min }}} \over {{I_{max}} + {I_{min}}}}$$ will be
16
A linear aperture whose width is 0.02 cm is placed immediately in front of a lens of focal length 60 cm. The aparture is illuminated normally by a parallel beam of wavelength 5 $$ \times $$ 10$$-$$5 cm. The distance of the first dark band of the diffraction pattern from the centre of the screen is
17
Electrons of mass m with de-Broglie wavelength $$\lambda $$ fall on the target in an X-ray tube. The cutoff wavelength ($$\lambda $$0) of the emitted X-ray is
18
Photons with energy 5 eV are incifent on a cathode C in a photoelectric cell. The maximum energy of emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode A, if the stopping potential of A relative to C is
19
If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd orbit, it emits a photon of wavelength $$\lambda $$. When it jumps from the 4th orbit to the 3rd orbit, the corresponding wavelength of the photon will be
20
The given circuit has two ideal diodes connected as shown in the figure. The current flowing through the resistance R1 will be


21
What is the output Y in the following circuit, when all the three inputs A, B, C are first 0 and then 1 ?


22
Starting from the centre of the earth having radius R, the variation of g (acceleration due to gravity) is shown by
23
Two cars P and Q start from a point at the same time in a straight line and their positions are represented by
xP(t) = (at + bt2) and xQ(t) = (ft $$-$$ t2).
At what time do the cars have the same velocity ?
xP(t) = (at + bt2) and xQ(t) = (ft $$-$$ t2).
At what time do the cars have the same velocity ?
24
In the given figure, a = 15 m s$$-$$2 represents the total acceleration of particle moving in the clockwise direction in a circle of radius R = 2.5 m at a given instant of time. The speed of the particle is


25
A rigid ball of mass m strikes a rigid wall at 60o and gets reflected without loss of speed as shown in the figure. The value of impulse imparted by the wall on the ball will be :


26
A bullet of mass 10 g moving horizontally with a velocity of 400 m s$$-$$1 strikes a wood block of mass 2 kg which is suspended by light inextensible string of length 5 m. As a result, the centre of gravity of the block found to rise a vertical distance of 10 cm. The speed of the bullet after it emerges out horizontally from the block will be
27
Two identical balls A and B having velocities of 0.5 m s$$-$$1 and $$-$$0.3 m s$$-$$1 respectively collide elastically in one dimension. The velocities of B and A after the collision respectively will be :
28
A particle moves from a point $$\left( { - 2\widehat i + 5\widehat j} \right)$$ to $$\left( {4\widehat j + 3\widehat k} \right)$$ when a force of $$\left( {4\widehat i + 3\widehat j} \right)$$ N is applied. How much work has been done by the force ?
29
Two rotating bodies A and B of masses m and 2m with moments of inertia $${I_A}$$ and $${I_B}$$ ($${I_B}$$ > $${I_A}$$) have equal kinetic energy of rotation. If LA and LB be their angular momenta respectively, then
30
A solid sphere of mass m and radius R is rotating about its diameter. A solid cylinder of same mass and same radius is also rotating about its geometrical axis with an angular speed twice that of the sphere. The ratio of their kinetic energies of rotation (Esphere / Ecylinder) will be
31
A light rod of length $$l$$ has two masses m1 and m2 attached to its two ends. The moment of inertia of the system about an axis perpendicular to the rod and passing through the centre of mass is
32
A satellite of mass m is orbiting the earth (of radius R) at a height h from its surface. The total energy of the satellite in terms of g0, the value of acceleration due to gravity at the earth's surface, is
33
Three liquids of densities $$\rho $$1, $$\rho $$2 and $$\rho $$3 (with $$\rho $$1 > $$\rho $$2 > $$\rho $$3), having the same value of surface tension T, rise to the same height in three identical capillaries. The angles of contact $$\theta $$1, $$\theta $$2 and $$\theta $$3 obey
34
Two identical bodies are made of a material for which the heat capacity increases with temperature. One of these is at 100oC, while the other one is at 0oC. If the two bodies are brought into contact, then, assuming no heat loss, the final common temperature is
35
A body cools from a temperature 3T to 2T in 10 minutes. The room temperature is T. Assume that Newton's law of cooling is applicable. The temperature of the body at the end of next 10 minutes will be
36
A rectangular film of liquid is extended from (4 cm $$ \times $$ 2 cm) to (5 cm $$ \times $$ 4 cm). If the work done is 3 $$ \times $$ 10$$-$$4 J, the value of the surface tension of the liquid is
37
One mole of an ideal monatomic gas undergoes a process described by the equation PV3 = constant. The heat capacity of the gas during this process is
38
The temperature inside a refrigerator is t2 oC. The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
39
A given sample of an ideal gas occupies a volume V at a pressure P and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas ?
40
A body of mass m is attached to the lower end of a spring whose upper end is fixed. The spring has negligible mass. When the mass m is slightly pulled down and released, it oscillates with a time period of 3s. When the mass m is increased by 1 kg, the time period of oscillations becomes 5 s. The value of m in kg is
41
The second overtone of an open organ pipe has the same frquency as the first overtone of a closed pipe L metre long. The length of the open pipe will be
42
Three sound waves of equal amplitudes have frequencies (n $$-$$ 1), n, (n + 1). They superimpose to give beats. The number of beats produced per second will be
43
Planck's constant (h), speed of light in vacuum (c) and Newton's gravitional constant (G) are three fundamental constants. Which of the following combinations of these has the dimension of length ?