Chemistry
The $E_{M^{3+} / M^{2+}}^o$ values for $\mathrm{Cr}, \mathrm{Mn}, \mathrm{Fe}$ and Co are $-0.41,+1.57,+0.77$ and +1.97 V respectively.
For which of these metals, the change in oxidation state from +2 to +3 is the easiest?
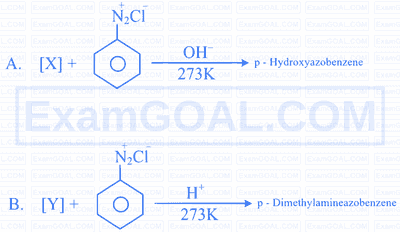
Complete the following 2 reactions A \& B by choosing appropriate reactants $[\mathrm{X}] \&[\mathrm{Y}]$.
Choose the statements which are incorrect in the case of Lanthanoids.
A. $\mathrm{Ce}^{4+}$ is diamagnetic while $\mathrm{Sm}^{3+}$ is paramagnetic.
B. The atomic size of the transition metals having atomic number greater than 71 are very close to that of the elements above them.
C. Lanthanoids react with hot water forming water soluble $\mathrm{Ln}(\mathrm{OH})_3$ with the liberation of $\mathrm{O}_2$.
D. The general electronic configuration of Lanthanoids is $(n-2) \mathrm{f}^{1-14} 5 \mathrm{~d}^0 6 \mathrm{~s}^2$, where $n=6$.
Given below are 2 statements: one Assertion and the other Reason. Which one of the following options is correct?
Assertion : Sulphur dioxide and Hydrogen peroxide can act as both oxidising and reducing agents but Nitric acid can act as only oxidising agent.
Reason : Sulphur and Oxygen can exhibit more than one stable oxidation states while Nitrogen does not.
From among the 4 given compounds identify the compounds which possess a net dipole moment.
A. cis-1,2-Dichloroethene
B. Tetrachloromethane
C. o-Dichlorobenzene
D. trans 2,3-Dibromobut-2-ene
Which is the correct order of increasing number of unpaired electrons in the following ions?
$$\mathrm{A}=\mathrm{Cr}^{2+}(\mathrm{Z}=24) \mathrm{B}=\mathrm{Cu}^{2+}(\mathrm{Z}=29) \quad \mathrm{C}=\mathrm{Ni}^{2+}(\mathrm{Z}=28) \mathrm{D}=\mathrm{Fe}^{3+}(\mathrm{Z}=26)$$
Identify X and Y formed in the following two reactions.
(i) Decan-1-ol $\xrightarrow{\text { Jones reagent }} \mathrm{X}$
(ii). Sodium salt of $\mathrm{X} \xrightarrow{\mathrm{NaOH} / \mathrm{CaO}, \Delta} \mathrm{Y}$
Match A, B, C and D with the appropriate functions given.
| Column I | Functions | ||
|---|---|---|---|
| A. | Oxidoreductase | P. | Malfunctioning leads to Addison's disease. |
| B. | Phosphodiester bonds | Q. | Regulates responses to external stimuli. |
| C. | Adrenal cortex | R. | Catalyses glycolysis. |
| D. | Epinephrine | S. | Links nucleotides together. |
Two statements, One Assertion and the other Reason, are given.
Which one of the following is the correct option?
Assertion: The acid strength of 4 compounds in the descending order is p- Nitrophenol > p-Methoxyphenol > Phenol > p-chlorophenol.
Reason: Electron withdrawing groups increase the acid strength while Electron donating groups decrease the acid strength of Phenol and its derivatives.
A. $=$ chromate $\mathrm{B}=$ permanganate $\mathrm{C}=$ dichromate
Which one of the following structures in Column I does not have the correct IUPAC name as given in Column II.
| S.No. | Structure | IUPAC name | |
|---|---|---|---|
| A. | $\left[\mathrm{CoBr}_2(\mathrm{en})_2\right] \mathrm{Cl}$ | P. | Dibromidodi-( ethan-1, 2 - diamine)cobalt (III) chloride |
| B. | $\mathrm{Na}\left[\mathrm{PtBrCl}\left(\mathrm{NO}_2\right)\left(\mathrm{NH}_3\right)\right]$ | Q. | Sodium amminebromidochloridonitrito- N - palatinate(II) |
| C. | $\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_2\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}\right] \mathrm{SO}_4$ | R. | Tetraamminediaquachloridochromium (III) sulphate. |
| D. | $\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_4\right]\left[\mathrm{PtCl}_4\right]$ | S. | Tetraammineplatinum (II) tetrachloridoplatinate (II) |
Identify the product $(\mathrm{Y})$ formed in the given reaction and the name of the reaction where $(\mathrm{X}) \rightarrow(\mathrm{Y})$.

(i) and (ii) are 2 chemical reactions carried out at TK.
(i). $\mathrm{X} \rightarrow \mathrm{Y}+\mathrm{W}$ with $\mathrm{k}_1$ as rate constant.
(ii). $\mathrm{X} \rightarrow \mathrm{Z}+\mathrm{W}$ with $k_2$ as rate constant.
The Activation energy for reaction (ii) is 3 times that of reaction (i). What is the expression for $k_1$ ?
Which one of the following is the major product formed when the given reaction occurs?

Identify the reagents to be used to complete the given reaction.
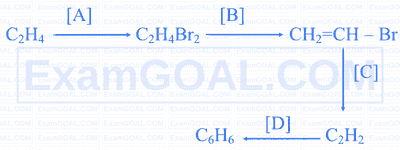
What is the standard electrode potential at 298 K for the reaction: $\mathrm{Cu}^{2+}+1 \mathrm{e}-\rightarrow \mathrm{Cu}^{+1}$ ?
Given: $\mathrm{E}_0 \mathrm{Cu}^{+1} / \mathrm{Cu}=0.5 \mathrm{~V} \quad \& \quad \mathrm{E}_0 \mathrm{Cu}^{+2} / \mathrm{Cu}=0.335 \mathrm{~V}$
The Standard Reduction potential at $25^{\circ} \mathrm{C}$ for $\left(\mathrm{MnO}_4\right)^{-1} / \mathrm{H}^{+}$is +1.49 V . The $\mathrm{E}^0$ values for four Metal ions :
(a). $\mathrm{Co}^{3+} / \mathrm{Co}^{2+}$
(b). $\mathrm{Cr}^{3+} / \mathrm{Cr}$
(c). $\mathrm{Au}^{3+} / \mathrm{Au}$ and
(d). $\mathrm{Ag}^{+} / \mathrm{Ag}$
are $+1.81 \mathrm{~V},-0.74 \mathrm{~V},+1.50 \mathrm{~V}$ and +0.8 V respectively. Identify two of them which cannot be oxidised by $\left(\mathrm{MnO}_4\right)^{-1} / \mathrm{H}^{+}$
Match the reactions in Column I with the correct products given in Column II
| S.No. | Column I | S.NO. | Column II |
|---|---|---|---|
| A. | 1- Chloropropane $+(\mathrm{Zn} / \mathrm{HCl}) \rightarrow$ | P. | Propanone + Methanal |
| B. | n- Hexane $+\left(\right.$ anh. $\left.\mathrm{AlCl}_3 / \mathrm{HCl}_{(\mathrm{g})}\right) \rightarrow$ | Q. | Propanone |
| C. | 2- Methylpropene $+\left(\mathrm{O}_3 \& \mathrm{Zn} / \mathrm{H}_2 \mathrm{O}\right) \rightarrow$ | R. | Propane |
| D. | Propyne $+\left(\mathrm{HgSO}_4 / \mathrm{H}_2 \mathrm{SO}_4\right.$ at 333 K$) \rightarrow$ | S. | 2-Methylpentane + 3-Methylpentane |
Two statements, one Assertion and the other Reason are given. Choose the correct option.
Assertion: Heterocyclic compounds like Pyridine and Thiophene are non-aromatic compounds.
Reason: According to Huckel rule for a given compound to exhibit aromaticity, the molecule must be planar, cyclic system having delocalised $(4 n+2) \pi$ electrons.
Match the reaction in Column I with the major product formed given in Column II.
| Column I - Reaction/conditions | Column II - Product | ||
|---|---|---|---|
| A. |  |
P. | Pentan-2-ol |
| B. |  |
Q. | 2-Hydroxybenzoic acid. |
| C. |  |
R. | Phenol |
| D. |  |
S. | 2-Methylpropan-1-ol |
Two statements, one Assertion and the other Reason are given. Choose the correct option.
Assertion: For strong electrolytes the plot of Molar conductivity versus Concentration gives a straight line with slope equal to +A and intercept equal to $\lambda_{\mathrm{m}}$
Reason: For strong electrolytes, $\lambda_{\mathrm{m}}$ increases slowly with dilution due to increase in the distance between the ions and increase in ionic mobility
Mathematics
Let $A=\{x: x=4 n+1, n \in Z, 0 \leq n<4\}$
$$\begin{aligned} & B=\{x: x=15 n+4, n \in N, n \leq 3\} \\ & C=\{x: x \text { is a prime number }, x \in A \cup B\} \end{aligned}$$
Then the cardinal number of set C is
If $y=\left(\sin ^{-1} x\right)^2+\left(\cos ^{-1} x\right)^2$,
then $\left(1-x^2\right) \frac{d^2 y}{d x^2}-x \frac{d y}{d x}=$
A bag contains $(n+1)$ coins. It is known that one of these coins has a head on both sides, whereas the other coins are fair. One of these coins is selected at random and tossed. If the probability that the toss results in heads is $\frac{7}{12}$, then the value of $n$ is :
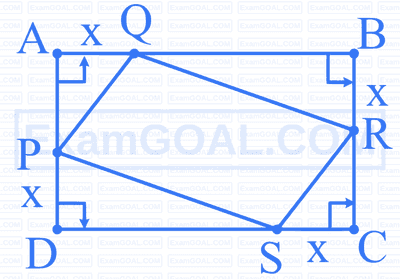
Quadrilateral PQRS is inscribed inside a rectangle of dimensions $10 \mathrm{~cm} \times 8 \mathrm{~cm}$. The value of ' $x$ ', if the area of the quadrilateral is minimum is
Simplified expression of
$1-\frac{\sin ^2 y}{1+\cos y}+\frac{1+\cos y}{\sin y}-\frac{\sin y}{1-\cos y}$ is :
Three bags contain a number of red and white balls are as follows.
Bag I: 3 red balls
Bag II: 2 red balls and 1 white ball
Bag III: 3 White balls
The probability that bag $i$ will be chosen and a ball is selected from it is $\frac{i}{6}, i=1,2,3$. If a white ball is selected, what is the probablity that it came from Bag III
The cost of 4 kg onion, 3 kg wheat and 2 kg rice is ₹ 500 .
The cost of 1 kg onion, 2 kg wheat and 3 kg rice is ₹ 300 .
The cost of 6 kg onion, 2 kg wheat and 3 kg rice is ₹ 575 .
The above situation can be represented in matrix form as $\mathrm{AX}=\mathrm{B}$. Then $\left|5 A^{-1}\right|=$
The inequality representing the following graph is

Physics

Three ideal diodes and resistors connected to the cell of negligible internal resistance is as shown. Find the current passing through the $10 \Omega$ resistor.
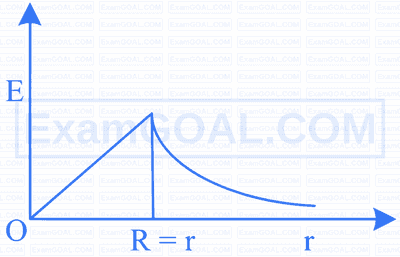
The electric field versus distance graph is shown as given. Select the correct statement from the following.
E- electric field
R-radius
r - distance from the centre