Identify the reagents to be used to complete the given reaction.
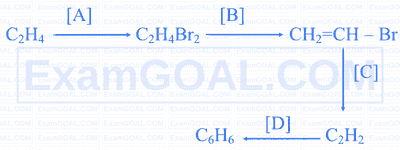
What is the standard electrode potential at 298 K for the reaction: $\mathrm{Cu}^{2+}+1 \mathrm{e}-\rightarrow \mathrm{Cu}^{+1}$ ?
Given: $\mathrm{E}_0 \mathrm{Cu}^{+1} / \mathrm{Cu}=0.5 \mathrm{~V} \quad \& \quad \mathrm{E}_0 \mathrm{Cu}^{+2} / \mathrm{Cu}=0.335 \mathrm{~V}$
The Standard Reduction potential at $25^{\circ} \mathrm{C}$ for $\left(\mathrm{MnO}_4\right)^{-1} / \mathrm{H}^{+}$is +1.49 V . The $\mathrm{E}^0$ values for four Metal ions :
(a). $\mathrm{Co}^{3+} / \mathrm{Co}^{2+}$
(b). $\mathrm{Cr}^{3+} / \mathrm{Cr}$
(c). $\mathrm{Au}^{3+} / \mathrm{Au}$ and
(d). $\mathrm{Ag}^{+} / \mathrm{Ag}$
are $+1.81 \mathrm{~V},-0.74 \mathrm{~V},+1.50 \mathrm{~V}$ and +0.8 V respectively. Identify two of them which cannot be oxidised by $\left(\mathrm{MnO}_4\right)^{-1} / \mathrm{H}^{+}$