Chemistry
1. An organic compound $\mathrm{A}\left(\mathrm{C}_5 \mathrm{H}_9 \mathrm{~N}\right)$ upon reaction with $\mathrm{Na} / \ma 2. The $E_{M^{3+} / M^{2+}}^o$ values for $\mathrm{Cr}, \mathrm{Mn}, \mathrm{Fe}$ and Co are $-0.41,+1.57,+0.77$ and +1.97 3. Given are 4 pairs of covalent molecules. Identify the pair in which both molecules have the same shape. 4. Lead storage battery contains $4.25 \mathrm{M} \mathrm{H}_2 \mathrm{SO}_4$ which has a density of $1.24 \mathrm{~g} / \m 5. Which of the following is the correct name according to IUPAC rules? 6. The Enthalpy of combustion of 1.0 mole of a reactive metal X at $27^{\circ} \mathrm{C}$ and 1.0 bar pressure to form a s 7. The ionisation constant of the weak acid HF whose concentration is 0.1 M is $3.5 \times 10^{-4}$ The Equilibrium constan 8. An Alkene " X " on reaction with hot acidified $\mathrm{KMnO}_4$ gave a mixture of Ethanoic acid and Propanone. Identify 9. Imagine an R - moiety of a pentapeptide molecule having one $-\mathrm{SH},-\mathrm{CONH}_2,-\mathrm{NH}_2$ groups each a 10. An aqueous solution which contains $42 \%$ by weight $(\mathrm{w} / \mathrm{W})$ of a volatile liquid "A" of molar mass 11. What would be the cell potential for the Galvanic cell which is represented by the electrochemical reaction:
$$
\begin{ 12. The hydrogenation of Ethyne is carried out at 600 K . The same reaction when carried out in presence of a catalyst maint 13. Complete the following 2 reactions A \& B by choosing appropriate reactants $[\mathrm{X}] \&[\mathrm{Y}]$.
14. The time needed for completion of $80 \%$ is $y$ times the half-life period of a first order reaction. What is the value 15. A current of 1.5 A is passed for 2 hours through an aqueous solution of $\mathrm{PdX} \mathrm{n}_{\mathrm{n}}$ where X i 16. Choose the statements which are incorrect in the case of Lanthanoids.
A. $\mathrm{Ce}^{4+}$ is diamagnetic while $\mathr 17. Larger number of oxidation states are exhibited by the actinoids than those of lanthanoids. The reason is:
18. Given below are 2 statements: one Assertion and the other Reason. Which one of the following options is correct?
Asserti 19. From among the 4 given compounds identify the compounds which possess a net dipole moment.
A. cis-1,2-Dichloroethene
B. 20. Which is the correct order of increasing number of unpaired electrons in the following ions?
$$\mathrm{A}=\mathrm{Cr}^{2 21. Which one of the following is incorrect?
22. A dilute solution of $\mathrm{K}_2 \mathrm{HgI}_4$ reagent is $95 \%$ ionised. What would be the approximate value of it 23. For a hypothetical chemical reaction $\mathrm{A}_2+3 \mathrm{~B}_2+$ Heat $\cdots\rightarrow 2 \mathrm{AB}_3$, which one 24. Choose the incorrect statement from the following.
25. Which one of the following compounds will give a yellow precipitate when reacted with $\mathrm{I}_2 / \mathrm{NaOH}$? 26. Identify X and Y formed in the following two reactions.
(i) Decan-1-ol $\xrightarrow{\text { Jones reagent }} \mathrm{X} 27. Choose the incorrect statement. 28. Match A, B, C and D with the appropriate functions given.
.tg {border-collapse:collapse;border-spacing:0;}
.tg td{bord 29. Two statements, One Assertion and the other Reason, are given.
Which one of the following is the correct option?
Asserti 30. Identify the incorrect statement. 31. Arrange the following ions in the decreasing order of covalent double bonds formed by the transition metal with oxygen.
32. Which one of the following structures in Column I does not have the correct IUPAC name as given in Column II.
.tg {bor 33. When $0.4 \mathrm{~g} \mathrm{CH}_3 \mathrm{COOH}$ is added to 40 g of Benzene to form a solution, the freezing point is 34. Two subatomic particles 1 and 2, with the same kinetic energies have their de- Broglie wavelengths as $\lambda_1 \& \lam 35. Identify the product $(\mathrm{Y})$ formed in the given reaction and the name of the reaction where $(\mathrm{X}) \right 36. Choose the correct statement:
37. A transition metal M forms 4 homoleptic octahedral coordination compounds, $\mathrm{A}, \mathrm{B}, \mathrm{C}$ and D of 38. Which one of the following is the correct statement?
39. (i) and (ii) are 2 chemical reactions carried out at TK.
(i). $\mathrm{X} \rightarrow \mathrm{Y}+\mathrm{W}$ with $\math 40. Which one of the following is the major product formed when the given reaction occurs?
41. Toluene when reacted with $\mathrm{Cl}_2$ gas at 385 K forms a product X which undergoes further reaction with Sodium et 42. A dilute solution of an ionic compound $\mathrm{A}_3 \mathrm{~B}$ has an Osmotic pressure which is 6 times that of $0.02 43. An organic compound " A " reacts with $\mathrm{Zn} / \mathrm{Hg}$ / Conc. HCl to form p-Xylene. It reduces Tollen's reag 44. The first ionisation enthalpy of Al in $\mathrm{kJ} \mathrm{mol}^{-1}$ is:
[Given the first ionisation enthalpy of $\mat 45. Choose the coordination compound which does not exhibit Optical activity.
46. Identify the reagents to be used to complete the given reaction.
47. What is the standard electrode potential at 298 K for the reaction: $\mathrm{Cu}^{2+}+1 \mathrm{e}-\rightarrow \mathrm{C 48. Which one of the following molecules attains greater stability on formation of its diatomic monovalent anion? 49. The Standard Reduction potential at $25^{\circ} \mathrm{C}$ for $\left(\mathrm{MnO}_4\right)^{-1} / \mathrm{H}^{+}$is +1 50. The best method for the separation of $o$-nitrophenol and $p$-nitrophenol from their mixture is :
51. For the reaction $\mathrm{A}_2+\mathrm{B}_2 \cdots \cdots>2 \mathrm{AB}, \Delta \mathrm{H}_{\mathrm{f}}=-400 \mathrm{~kJ 52. The correct order of spin only magnetic moments among the following is:
[Given: Atomic numbers: $\mathrm{Mn}=25, \mathrm 53. Choose the incorrect statement from the following.
54. Match the reactions in Column I with the correct products given in Column II
.tg {border-collapse:collapse;border-spac 55. The rate constant for a zero order reaction $\mathrm{A} \rightarrow \mathrm{B}+\mathrm{C}$ is $6.0 \times 10^{-3} \mathr 56. When one mole of a hydrocarbon $\mathbf{C}_{\mathbf{a}} \mathbf{H}_{\mathbf{b}}$ undergoes complete combustion, it requi 57. For the reaction $\mathrm{P}+\mathrm{Q} \leq \mathrm{R}+\mathrm{S}$, carried out at 298 K , the equilibrium constant was 58. Two statements, one Assertion and the other Reason are given. Choose the correct option.
Assertion: Heterocyclic compoun 59. Match the reaction in Column I with the major product formed given in Column II.
.tg {border-collapse:collapse;border- 60. Two statements, one Assertion and the other Reason are given. Choose the correct option.
Assertion: For strong electroly
Mathematics
1. The terms of an infinitely decreasing geometric progression in which all the terms are positive, the first term is $\mat 2. For real numbers $x$ and $y, x R y \Leftrightarrow x-y+\sqrt{2}$ is an irrational number. Then the relation R is:
3. Let $A=\{x: x=4 n+1, n \in Z, 0 \leq n
$$\begin{aligned}
& B=\{x: x=15 n+4, n \in N, n \leq 3\} \\
& C=\{x: x \text { is 4. If $ 2 y=\left[\cot ^{-1}\left(\frac{\sqrt{3} \cos x+\sin x}{\cos x-\sqrt{3} \sin x}\right)\right]^2 \forall x \in\left( 5. Five persons entered the lift cabin on the ground floor of an eight-floor apartment. Suppose that each of them independe 6. If $\cos A=\frac{3}{4}$, then $\left(32 \sin \frac{A}{2} \sin \frac{5 A}{2}\right)=$ 7. If $A=\frac{1}{\pi}\left[\begin{array}{cc}\sin ^{-1} \frac{1}{2} & \tan ^{-1} \frac{x}{\pi} \\ \sin ^{-1} \frac{x}{\pi} 8. The length of the latus rectum of a conic $49 y^2-16 x^2=784$ is
9. The curve $4 y=3 x^4-2 x^2$ attains ----------- at the points $x=-\frac{1}{\sqrt{3}}$ and $x=\frac{1}{\sqrt{3}}$
10. The area of the region enclosed by the lines $2 x+y=10, y=1, y=5$ and the $y$-axis is
11. $x=a(\theta+\sin \theta)$ and $y=a(1-\cos \theta)$ represents the equation of a curve. If $\theta$ changes at a constant 12. The function $f(x)=\left\{\begin{array}{l}\frac{|x|}{x}, \text { if } x \neq 0 \\ 0, \text { if } x=0\end{array}\right.$ 13. Integrating factor of the differential equation $\frac{d y}{d x}+y=\frac{x^3+y}{x}$ is
14. If $y=\left(\sin ^{-1} x\right)^2+\left(\cos ^{-1} x\right)^2$,
then $\left(1-x^2\right) \frac{d^2 y}{d x^2}-x \frac{d 15. If ${ }^{n+2} C_8:{ }^{n-2} P_4=57: 16$, then ' $n$ ' is 16. Differentiate $\log _a x$ with respect to $a^x$
17. On each working day of a school there are six periods. The number of ways in which five subjects are arranged if each su 18. A bag contains $(n+1)$ coins. It is known that one of these coins has a head on both sides, whereas the other coins are 19. Quadrilateral PQRS is inscribed inside a rectangle of dimensions $10 \mathrm{~cm} \times 8 \mathrm{~cm}$. The value of ' 20. If $A=\left[\begin{array}{ccc}0 & -1 & 2 \\ 1 & 0 & 3 \\ -2 & -3 & 0\end{array}\right]$, then $A+2 A^T=$
21. Simplified expression of
$1-\frac{\sin ^2 y}{1+\cos y}+\frac{1+\cos y}{\sin y}-\frac{\sin y}{1-\cos y}$ is : 22. The length of the perpendicular from the point $P(1,-1,2)$ to the given line $\frac{x+1}{2}=\frac{y-2}{-3}=\frac{z+2}{4} 23. The point on the line $x+y=4$ that lie at a unit distance from the line $4 x+3 y=10$ is
24. The area of the region bounded by the ellipse $\frac{x^2}{a^2}+\frac{y^2}{b^2}=1$ is
25. If $A(\operatorname{adj} A)=\left[\begin{array}{lll}5 & 0 & 0 \\ 0 & 5 & 0 \\ 0 & 0 & 5\end{array}\right]$, then the val 26. In a kabaddi league, two matches are being played between Jaipur and Delhi. It is assumed that the outcomes of the two g 27. $\int \frac{\sin x+\cos x}{\sqrt{1+2 \sin x \cos x}} d x=\varphi(x)+C$ Then $\varphi(x)=$
28. If the mean of $4,7,2,8,6$ and $k$ is 7 . Then the mean deviation from the mean of these observations is 29. $\int\limits_0^{\frac{\pi}{2}} \log \left(\frac{5+4 \sin x}{5+4 \cos x}\right) d x=$ 30. The degree of the differential equation $\left[1+\left(\frac{d y}{d x}\right)^2\right]^{\frac{3}{4}}=\left(\frac{d^2 y}{ 31. Evaluate the value of $(1.02)^8$ using binomial theorem up to two decimal places. 32. $\int \frac{e^{\tan ^{-1} x}}{\left(1+x^2\right)}\left(1+x+x^2\right) d x=$ 33. Which of the following transformations reduce the differential equation $\frac{d z}{d x}+\frac{z}{x} \log z=\frac{z}{x^2 34. The least area of a circle circumscribing any right-angle triangle of area $\frac{9}{\pi}$ sq units is
35. $\lim _\limits{\theta \rightarrow \frac{\pi}{2}} \frac{1-\sin \theta}{\left(\frac{\pi}{2}-\theta\right) \cos \theta}$ is 36. If for two events $A$ and $B, P(A-B)=\frac{1}{5}$ and $P(A)=\frac{3}{5}$ then $P(B / A)=$
37. $\int \frac{x}{(x-1)(x-2)^2} d x=a \log \left|\frac{x-1}{x-2}\right|+\frac{b}{(x-2)}+c$ then
38. The complex number $\frac{1+7 i}{(2-i)^2}$ lies in
39. If a function $f:[2, \infty) \rightarrow R$ defined by $f(x)=x^2-4 x+5$, then the range of $f$ is
40. The corner points of the feasible region determined by the system of linear constraints are $(0,3),(1,1)$ and $(3,0)$, I 41. Three bags contain a number of red and white balls are as follows.
Bag I: 3 red balls
Bag II: 2 red balls and 1 white ba 42. The line $A B$ passes through the point $P(-4,3)$ and the portion of the line intercepted between the axes is divided in 43. If $Q(1,0,1)$ is the image of the point $P(a, b, c)$ in the line $\frac{x+1}{2}=\frac{y-3}{-2}=\frac{z}{-1}$ then $a+b+c 44. The cost of 4 kg onion, 3 kg wheat and 2 kg rice is ₹ 500 .
The cost of 1 kg onion, 2 kg wheat and 3 kg rice is ₹ 300 .
45. Let $A$ and $G$ denote the arithmetic mean and geometric mean of positive real numbers $5^x$ and $5^{1-x}$. Then the min 46. In a triangle $A B C$ the coordinate of the vertex $A$ is $(1,2)$. Equations of the median through $B$ and $C$ are respe 47. The radius of the circle passes through the foci of a conic $\frac{x^2}{16}+\frac{y^2}{9}=1$ and has its centre at $(0,3 48. If $A=\{1,2,4\} \quad B=\{2,4,5\} \quad C=\{2,5\}$ then $(A-B) \cap(B-C)=$
49. $\int \frac{d x}{x \sqrt{4 x^2-9}}=$ 50. In the interval $(0,1)$ the function $f(x)=x^2-x+1$ is
51. If $|\vec{a}|=2 \sqrt{2}$ and $|\vec{b}|=3$ and angle between $\vec{a}$ and $\vec{b}$ is $\frac{\pi}{4}$. If a parallelo 52. The value of $\tan ^{-1}\left(\tan \frac{7 \pi}{6}\right)$ is
53. The cofactor of the element $a_{21}$ in the expansion of $\Delta=\left|\begin{array}{ccc}1 & 4 & 4 \\ -3 & 5 & 9 \\ 2 & 54. If for real values of $x, \cos \theta=x+\frac{1}{x}$, then $X$
55. Solution of the differential equation $y \frac{d y}{d x}+x=0$ represents a family of
56. The inequality representing the following graph is
57. Position vector of P and Q are $\hat{\imath}+3 \hat{\jmath}-7 \hat{k}$ and $5 \hat{\imath}-2 \hat{\jmath}+4 \hat{k}$ res 58. Domain of the function $f(x)=\sqrt{\sin ^{-1}(2 x)+\frac{\pi}{6}}$ for real valued of $x$ is
59. Shortest distance between the lines $\vec{r}=(8+3 \lambda) \hat{\imath}-(9+16 \lambda) \hat{\jmath}+(10+7 \lambda) \hat{ 60. $\lim _\limits{x \rightarrow 1} \frac{(\sqrt{x}-1)(2 x-3)}{2 x^2+x-3}$ is
Physics
1. A circular coil of area $2 \sqrt{2} \mathrm{~cm}^2$ and resistance $2 \boldsymbol{\Omega}$ is arranged vertically in the 2. A car, starting from rest, accelerates at the rate (f) through a distance ( $S$ ), then continues at constant speed for 3. A particle ' X ' carrying a charge +Q is moving in a circular path of radius R around another particle ' Y ' having a ch 4.
Three ideal diodes and resistors connected to the cell of negligible internal resistance is as shown. Find the current 5. A coin is placed on a disc rotating with an angular velocity $\omega$. The co-efficient of friction between the disc and 6. A beam of light parallel to the principal axis of a concave and convex lens, first passes through the concave lens of fo 7. Three point charges $-1 C,+1 C,+1 C$ are placed at points $A, B, C$ respectively of a triangle $A B C$. What is the tota 8. When a body of refractive index $\boldsymbol{\mu}=\mathbf{1 . 4}$ is put into a liquid, the body becomes invisible. What 9. What is the frequency ' $\nu$ ' of the electron in Bohr's first orbit of radius ' $r$ ' of the hydrogen atom? 10. Water from a tap emerges vertically downwards with an initial speed of $1.0 \mathrm{~ms}^{-1}$. The cross-sectional area 11. A bullet of momentum $p$ is fired into a door and gets embedded exactly at the center of the door. The door is 1.0 m wid 12. A wind mill converts a fixed fraction of the wind energy intercepted by its blades into electrical energy. The electrica 13. The value of universal gravitational constant was first determined by 14. A current of 2 A is passed through the primary coil. The total flux linked with the secondary coil, which is closely wou 15. To get 300 MW electric power for half an hour, how much mass is to be completely converted into energy? 16. The electric field versus distance graph is shown as given. Select the correct statement from the following.
E- electric 17. One end of a nylon rope of length 1 and diameter 10 mm is fixed to free limb. A monkey weighing 100 N jumps to catch the 18. Two spherical planets P and Q have the same uniform density $\rho$, and masses Mp and $\mathrm{MQ}_{\mathrm{Q}}$ and sur 19. What is the dimensional formula for electric flux? 20. The ratio of the number of turns of the primary coil to the secondary coil of an ideal transformer is $5: 1$. The primar 21. The wire made of Phosphor bronze is used as the suspension strip in the moving coil galvanometer, because: 22. Fusion reaction is more energetic than fission reaction because 23. Which physical quantity has the unit joule / tesla? 24. In to a uniform transverse magnetic field, two charged particles having the same mass and charge enter and move in two d 25. The heat required to increase the temperature of 4 moles of a mono-atomic ideal gas from $273^{\circ} \mathrm{C}$ to $47 26. Rods $A$ and $B$ have their lengths in the ratio $1: 2$. Their thermal conductivities are $K_1$ and $K_2$ respectively. 27. The square of resultant of two equal electric field vectors is three times their product. Angle between them is 28. The nucleus of oxygen atom contains 8 protons and 8 neutrons. What is the mass defect in amu?
[Given Mass of proton $=1. 29. A monoatomic ideal gas, initially at temperature $T_1$, is enclosed in a cylinder fitted with a frictionless piston. The 30. Time period of oscillation of a mass suspended from a spring is T . If the spring is cut into four equal parts and the s 31. Ten cells, each having internal resistance $1 \Omega$ and emf 1.5 V are connected in series. But unknowingly 3 cells are 32. A galvanometer of $50 \Omega$ resistance is converted into an ammeter using a shunt resistance of $10 \Omega$. If the sa 33. A gun is used to fire a bullet at an angle of $30^{\circ}$ and at $60^{\circ}$ respectively. The ratios of heights and R 34. Diamond is considered as an insulator because
35. A ray of light is travelling from glass of refractive index $\frac{3}{2}$ to water of refractive Index $\frac{4}{3}$. Wh 36. An ideal gas is expanding such that $P T^2=$ constant. The coefficient of volume expansion of the gas is 37. The focal lengths of the objective and eyepiece of a compound microscope are 1 cm and 10 cm respectively. Length of the 38. If the radius of earth were to shrink by two percent, its mass remaining the same, the acceleration due to gravity on th 39. The resistance of the conductor is $\sqrt{3} \Omega$ the angle made by the V-I graph with the voltage axis is $\theta$ 40. A body weighing 125 kg just slides down a rough inclined plane that rises 1 m in every 2 m . What is the coefficient of 41. In which of the following circuit do we find the current and voltage in phase? 42. When a dielectric slab of dielectric constant $\mathrm{k}=2$ is used to fill the space between the plates of a parallel 43. de Broglie wave length associated with an electron accelerated through a potential difference ' V ' is ' $\lambda$ '. If 44. An open pipe is in second harmonic with frequency $f_1$. One end of the tube is closed and frequency is increased to $f_ 45. Across the 220 V source of internal resistance $20 \Omega$, how many lamps of $40 \mathrm{~W}, 100 \mathrm{~V}$ can be c 46. In the expression $\mathrm{P}=\mathrm{El}^2 \mathrm{~m}^{-5} \mathrm{G}^{-2}$ where $\mathrm{E}, 1, \mathrm{~m}$ and G r 47. The separation between C and O atoms in CO is 0.12 nm . The distance of C atom from the centre of mass is 48. An EM wave of frequency $5 \times 10^9 \mathrm{~Hz}$ falls normally on a rectangular slit of width 3 cm . What is the to 49. Two wires A and B made of same material having length 10 cm and 40 cm respectively are connected in parallel to the same 50. When a metallic spherical shell of radius 20 cm is charged, the potential on its surface is found to be 5 V . The potent 51. What is the maximum wave length of EM radiation required to move an electron from the valance band to conduction band of 52. What should be the value of the inductance of the coil which is to be connected to 220 V , 50 Hz supply so that maximum 53. In the Young's double slit experiment, when a monochromatic light is used, fringe width obtained is 1 mm . If the wave l 54. A monochromatic light of wave length $6000^{\circ} \mathrm{A}$ is passed through two media A and B of thickness 10 cm an 55. By connecting two given capacitors, a technician was able to make two new capacitors having the effective capacitance $1 56. A rectangular coil of length 10 cm and breadth 9 cm carries a current of 10 A . A long straight conductor carrying a cur 57. For a short magnet, the magnetic field on the axial line at a distance 10 cm from its centre is $1.6 \mathrm{~m} \times 58. What is the kinetic energy of the electron in the nth level, moving in a plane under the influence of a magnetic field ' 59. Which of the following statement is wrong regarding the photo electric effect.
60. What is the velocity of light in vacuum if the velocity of light in a medium of refractive index 1.2 is ' v ' $\mathrm{m
1
COMEDK 2025 Afternoon Shift
MCQ (Single Correct Answer)
+1
-0
Which of the following is the correct name according to IUPAC rules?
A

B

C
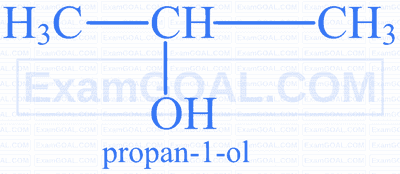
D

2
COMEDK 2025 Afternoon Shift
MCQ (Single Correct Answer)
+1
-0
The Enthalpy of combustion of 1.0 mole of a reactive metal X at $27^{\circ} \mathrm{C}$ and 1.0 bar pressure to form a solid oxide ( XO ) is $-601.83 \mathrm{~kJ} / \mathrm{mol}$. The Internal energy change for this reaction is ___________ kJ. $$\left(\mathrm{R}=8.314 \mathrm{j} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right)$$
A
701.33
B
754.58
C
$-$614.30
D
$-$600.58
3
COMEDK 2025 Afternoon Shift
MCQ (Single Correct Answer)
+1
-0
The ionisation constant of the weak acid HF whose concentration is 0.1 M is $3.5 \times 10^{-4}$ The Equilibrium constant value for the reaction $\mathrm{F}^{-}+\mathrm{H}_2 \mathrm{O} \leftrightharpoons \mathrm{HF}+\mathrm{OH}^{-}$is _________ and the pH of aqueous solution of the weak acid is __________ .
A
$\mathrm{K}_{\mathrm{eq}}=4.4 \times 10^{-11} \& \mathrm{pH}=4.25$
B
$\mathrm{K}_{\mathrm{eq}}=3.58 \times 10^{-11} \& \mathrm{pH}=4.19$
C
$\mathrm{K}_{\mathrm{eq}}=2.86 \times 10^{-11} \& \mathrm{pH}=3.23$
D
$\mathrm{K}_{\mathrm{eq}}=3.92 \times 10^{-10} \& \mathrm{pH}=4.07$
4
COMEDK 2025 Afternoon Shift
MCQ (Single Correct Answer)
+1
-0
An Alkene " X " on reaction with hot acidified $\mathrm{KMnO}_4$ gave a mixture of Ethanoic acid and Propanone. Identify " X ".
A
Pent-2-ene
B
2-Methylbut-2-ene.
C
But-2-ene
D
2,3 -Dimethylbut-2-ene
Paper analysis
Total Questions
Chemistry
60
Mathematics
60
Physics
60
COMEDK
Papers
2025
2024
2022
2021
2020