AIPMT 2011 Prelims
Paper was held on
Sun, Apr 3, 2011 10:00 AM
Biology
1
When a neuron is in resting state i.e., not conducting any impulse, the axonal membrane is
2
A large proportion of oxygen remains unused in the human blood even after its uptake by the body tissues. This O2
3
'Bundle of his' is a part of which one of the following organs in humans?
4
Which one of the following plasma proteins is involved in the coagulation of blood?
5
Arteries are best defined as the vessels which
6
A person with unknown blood group under ABO system, has suffered much blood loss in an accident and needs immediate blood transfusion. His friend who has valid certificate of his own blood type, offers for blood donation without delay. What would have been the type of blood group of the donar friend?
7
Which one of the following statements is correct regarding blood pressure?
8
Which one of the following correctly explains the function of a specific part of the human nephron?
9
Which one of the following is not a part of a renal pyramid?
10
Which one of the following statements is correct with respect to kidney function regulation?
11
Three of the following pairs of the human skeletal parts are correctly matched with their respective inclusive skeletel category and one pair is not matched. Identify the non-matching pair.
12
CAM helps the plants in
13
Match the source gland with its respective hormone and function and select the correct option.
14
Given below is an incomplete table on hormones, their source glands and one major effect of each human body. Identify the option representing correct grouping of hormone its gland and effect.
| Gland | Secretion | Effect on body |
|---|---|---|
| A | Estrogen | Maintenance of secondary sexual characters |
| Alpha cells of Islets of Langerhans |
B | Raises blood sugar level |
| Anterior pituitary | C | Over secretion leads to gigantism |
15
Filiform apparatus is a characteristic feature of
16
Nucellar polyembryony is reported in species of
17
Which one of the following pollinations is autogamous?
18
The ''eyes'' of the potato tuber are
19
Wind pollination is common in
20
If for some reason, the vasa efferentia in the
human reproductive system get blocked, the
gametes will not be transported from :
21
The testes in humans are situated outside the
abdominal cavity inside a pouch called scrotum.
The purpose served is for :
22
The figure given below depicts a diagrammatic
sectional view of the female reproductive system
of humans. Which one set of three parts out of I-VI have been correctly identified?


23
Which one of the following is the most widely
accepted method of contraception in India, as at
present ?
24
Flowers are zygomorphic in
25
Which one of the following also acts as a catalyst in a bacterial cell?
26
Which one of the following organisms is not an eukaryotic cells?
27
In eubacteria, a cellular component that resembles eukaryotic cell is
28
Which one of the following is incorrectly matched?
29
The gametophyte is not an independent, free-living generation in
30
Compared with the gametophytes of the bryophytes, the gametophytes of vascular plants tend to be
31
Archegoniophore is present in
32
A prokaryotic autotrophic nitrogen fixing symbiont is found in
33
What will you look for to identify the sex of the following?
34
Which one of the following groups of animals is correctly matched with its characteristic feature without any exception?
35
In which one of the following the genus name, its two characters and its class/phylun are correctly matched?
36
Which one of the following statements is correct?
37
Medical Termination of Pregnancy (MTP) is
considered safe up to how many weeks of
pregnancy ?
38
The ovary is half inferior in flowers of
39
A drupe develops in
40
The correct floral formula of chilli is
41
The curve given below shows enzymatic activity in relation to three conditions (pH, temperature and substrate concentration). What do the two axes (x and y) represent?


42
Which one of the following structural formulae of two organic compounds is correctly identified along with its related function?


43
Select the correct option with respect to mitosis.
44
One very special feature in the earthworm
pheretima is that :
45
Which one of the following statements is
correct for secondary succession?
46
Of the total incident solar radiation the
proportion of PAR is
47
Mass of living matter at a trophic level in an
area at any time is called :
48
Which one of the following statements for
pyramid of energy is incorrect, whereas the
remaining three are correct?
49
Which of the following has maximum genetic
diversity in India?
50
Which one of the following have the highest
number of species in nature ?
51
The cork cambium, cork and secondary cortex
are collectively called :
52
Ground tissue includes :
53
Which one of the following is categorised as a
parasite in true sense ?
54
Which of the following is correctly stated as it
happens in the common cockroach ?
55
The ciliated columnar epithelial cells in humans
are known to occur in :
56
Important site for formation of glycoproteins and
glycolipids is :
57
Peptide synthesis inside a cell takes place in :
58
The figure given below shows a small part of
human lung where exchange of gases takes
place. Select the option which represents labelled
part (A, B, C or D) correctly identified along
with its function.


59
Mutations can be induced with :
60
What would be the number of chromosomes
of the aleurone cells of a plant with 42
chromosomes in its root tip cells?
61
Which one of the following acts as a
physiological barrier to the entry of microorganisms in human body?
62
At which stage of HIV infection does one
usually show symptoms of AIDS?
63
A certain patient is suspected to be suffering
from Acquired Immuno Deficiency
Syndrome. Which diagnostic technique will
you recommend for its detection?
64
The most common substrate used in distilleries
for the production of ethanol is :
65
When two unrelated individuals or lines are
crossed, the per romance of F1 hybrid is often
superior to both parents. This phenomenon is
called :
66
Which one of the following conditions correctly
describes the manner of determining the sex ?
67
What are those structures that appear as 'beads-on-string' in the chromosomes when viewed
under electron microscope ?
68
What was the most significant trend in the evolution of modern man (Homo sapiens) from his
ancestors ?
69
Where will you look for the sporozoites of the
malarial parasite ?
70
Continuous addition of sugars in 'fed batch'
fermentation is done to :
71
An organism used as a Biofertilizer for raising
soyabean crop is :
72
Which of the following is mainly produced by
the activity of anaerobic bacteria on sewage ?
73
Which one of the following is not a biofertilizer ?
74
Which one of the following animals is correctly matched with its particular taxonomic category?
75
Secondary sewage treatment is mainly a :
76
Ethanol is commercially produced through a
particular speciesl of :
77
Organisms called Methanogens are most
abundant in a :
78
Given below is a sample of a portion of DNA
strand giving the base sequence on the opposite
strands. What is so special shown in it ?
5' .............. GAATTC ................ 3'
3' .............. CTTAAG ................. 5'
5' .............. GAATTC ................ 3'
3' .............. CTTAAG ................. 5'
79
Agarose extracted from sea weeds finds use in :
80
There is a restriction endonuclease called
EcoRl. What does "co" part in it stand for ?
81
The process of RNA interference has been used
in the development of plants resistant to :
82
Maximum number of existing transgenic animals
is of :
83
What type of human population is represented
by the following age pyramid ?


84
Large Woody Vines are more commonly found in
85
Consider the following four conditions (a – d)
and select the correct pair of them as adaptation
to environment in desert lizards.
The conditions :
(a) burrowing in soil to escape high temperature
(b) losing heat rapidly from the body during high temperature
(c) bask in sun when temperature is low
(d) insulating body due to thick fatty dermis
The conditions :
(a) burrowing in soil to escape high temperature
(b) losing heat rapidly from the body during high temperature
(c) bask in sun when temperature is low
(d) insulating body due to thick fatty dermis
Chemistry
1
The total number of atomic orbitals in fourth energy level of an atom is
2
The energies E1 and E2 of two radiations are 25 eV and 50 eV respectively. The relation between their wavelengths i.e., $$\lambda $$1 and $$\lambda $$2 will be
3
In a set of reactions m-bromobenzoic acid gave a product D. Identify the product D.


4
What is the product obtained in the following reaction?


5
Which one of the following statements is not true regarding (+) lactose?
6
The freezing point depression constant for water is $$-$$1.86o C m$$-$$1. If 5.00 g Na2SO4 is dissolved in 45.0 g H2O, the freezing point is changed by $$-$$3.82oC. Calculate the van't Hoff factor for Na2SO4.
7
The van't Hoff factor $$i$$ for a compound which undergoes dissociation in one solvent and association in other solvent is respectively
8
Standard electrode potential of three metals X, Y and Z are $$-$$1.2 V, + 0.5 V and $$-$$ 3.0 V respectively. The reducing power of these metals will be
9
Standard electrode potential for Sn4+/Sn2+ couple is + 0.15 V and that for the Cr3+/Cr couple is $$-$$ 0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be
10
The electrode potentials for Cu2+(aq) + e$$-$$ $$ \to $$ Cu+(aq)
and Cu+(aq) + e$$-$$ $$ \to $$ Cu(s) are + 0.15 V and + 0.50 V respectively.
The value of Eocu2+/cu will be
and Cu+(aq) + e$$-$$ $$ \to $$ Cu(s) are + 0.15 V and + 0.50 V respectively.
The value of Eocu2+/cu will be
11
Which one of the following statements for the order of a reaction is incorrect?
12
Name the two type of the structure of silicate in which one oxygen atom of [SiO4]4$$-$$ is shared?
13
Clemmensen reduction of a ketone is carried out in the presence of which of the following?
14
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of + 2 oxidation state will be there in which of the following order?
(At. nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
(At. nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
15
Acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of
16
The complex, [Pt(Py)(NH3)BrCl] will have how many geometrical isomers ?
17
The d-electron configurations of Cr2+, Mn2+, Fe2+ and Co2+ are d4, d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
(At. Nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
(At. Nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
18
The complexes [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the examples of which type of isomerism?
19
Of the following complex ions, which is diamagnetic in nature?
20
Which of the following carbonyls will have the strongest C $$-$$ O bond?
21
Which of the following complex compounds will exhibit highest paramagnetic behaviour?
22
A buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of NH+4 is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 $$ \times $$ 10$$-$$5, what is the pH of this solution? (log 2.7 = 0.43)
23
If n = 6, the correct sequence for filling of electrons will be
24
Which of the following has the minimum bond length ?
25
The correct order of increasing bond length of C $$-$$ H, C $$-$$ O, C $$-$$ C and C$$=$$C is
26
Which of the two ions from the list given below that have the geometry that is explained by the same hybridization of orbitals, NO$$_2^ - $$. NO$$_3^ - $$, NH$$_2^ - $$, NH$$_4^ + $$, SCN$$-$$?
27
The pairs of species of oxygen and their magnetic behavior are noted below. Which of the following presents the correct description?
28
If the enthalpy change for the transition of liquid water to steam is 30 kJ mol$$-$$1 at 27oC, the entropy change for the process would be
29
Enthalpy change for the reaction,
4H(g) $$ \to $$ 2H2(g) is $$-$$869.6 kJ
The dissociation energy of H $$-$$ H bond is
4H(g) $$ \to $$ 2H2(g) is $$-$$869.6 kJ
The dissociation energy of H $$-$$ H bond is
30
Which of the following is correct option for free expansion of an ideal gas under adiabatic condition ?
31
The value of $$\Delta $$H for the reaction
X2(g) + 4Y2(g) $$\rightleftharpoons$$ 2XY4(g)
is less than zero. Formation of XY4(g) will be favoured at
X2(g) + 4Y2(g) $$\rightleftharpoons$$ 2XY4(g)
is less than zero. Formation of XY4(g) will be favoured at
32
For the reaction, N2(g) + O2(g) $$\rightleftharpoons$$ 2NO(g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction,
2NO(g) + O2(g) $$\rightleftharpoons$$ 2NO2(g)
What is K for the reaction,
NO2(g) $$\rightleftharpoons$$ $${1 \over 2}$$N2(g) + O2(g)
2NO(g) + O2(g) $$\rightleftharpoons$$ 2NO2(g)
What is K for the reaction,
NO2(g) $$\rightleftharpoons$$ $${1 \over 2}$$N2(g) + O2(g)
33
Which of the following is least likely to behave as Lewis base?
34
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear ?
35
The correct IUPAC name for the compound is


36
Which one of the following is most reactive towards electrophilic reagent?
37
Which one is a nucleophilic substitution reaction among the following ?
38
The Lassaigne's extract is boiled with conc. HNO3 while testing for halogens. By doing so it
39
In the following reactions,

the major products (A) and (C) are respectively

the major products (A) and (C) are respectively
Physics
1
In photoelectric emission process from a metal of work function 1.8 eV, the kinetic energy of most energetic electrons is 0.5 eV. The corresponding stopping potential is
2
There are four light-weight-rod samples A, B, C, D separately suspended by threads. A bar magnet is slowly brought near each sample and the following observations are noted
(i) A is feebly repelled
(ii) B is feebly attracted
(iii) C is strongly attracted
(iv) D remains unaffected
Which one of the following is true ?
(i) A is feebly repelled
(ii) B is feebly attracted
(iii) C is strongly attracted
(iv) D remains unaffected
Which one of the following is true ?
3
An ac voltage is applied to a resistance R and an inductor L in series. If R and the inductive reactance are both equal to 3 $$\Omega $$, the phase difference between the applied voltage and the current in the circuit is
4
In the ac circuit an alternating voltage e = $$200\sqrt 2 $$sin100t volts is connected to a capacitor of capacity 1 $$\mu $$F. The r.m.s. value of the current in the circuit is
5
The current $$i$$ in a coil varies with time as shown in the figure. The variation of induced emf with time would be


6
The electric and the magnetic field, associated with an e.m. wave, propagating along the +z-axis, can be represented by
7
The decreasing order of wavelength of infrared, microwave, ultraviolet and gamma rays is
8
Which of the following is not due to total internal reflection ?
9
A biconvex lens has a radius of curvature of magnitude 20 cm. Which one of the following options describe best the image formed of an object of height 2 cm placed 30 cm from the lens ?
10
Photoelectric emission occurs only when the incident light has more than a certain minimum
11
Light of two different frequencies whose photons have energies 1 eV and 2.5 eV respectively illuminate a metallic surface whose work function is 0.5 eV successively. Ratio of maximum speeds of emitted electrons will be
12
Electrons used in an electron microscope are accelerated by a voltage of 25 kV. If the voltage is increased to 100 kV then the de-Broglie wavelength associated with the electrons would
13
A current carrying closed loop in the form of a right angle isosceles triangle ABC is placed in uniform magnetic field acting along AB. If the magnetic force on the arm BC is $$\overrightarrow {F,} $$ the force on the arm AC is


14
The wavelength of the first line of Lyman series for hydrogen atom is equal to that of the second line of Balmer series for a hydrogen like ion. The atomic number Z of hydrogen like ion is
15
The power obtained in a reactor using U235 disintegration is 1000 kW. The mass decay of U235 per hour is
16
Fusion reaction takes place at high temperature because
17
Symbolic representation of four logic gates are shown as

Pick out which ones are for AND, NAND and NOT gates, respectively

Pick out which ones are for AND, NAND and NOT gates, respectively
18
If a small amount of antimony is added to germanium crystal
19
In forward biasing of the p-n junction
20
A planet moving along an elliptical orbit is closest to the sun at a distance r1 and farthest away at a distance of r2. If $$v$$1 and $$v$$2 are the linear velocities at these points respectively, then the ratio $${{{v_1}} \over {{v_2}}}$$ is
21
A boy standing at the top of a tower of 20 m height drops a stone. Assuming g = 10 m s$$-$$2, the velocity with which it hits the ground is
22
A particle moves in a circle of radius 5 cm with constant speed and time period 0.2$$\pi $$ s. The acceleration of the particle is
23
A missile is fired for maximum range with an initial velocity of 20 m/s. If g = 10 m/s2, the range of the missile is
24
A body is moving with velocity 30 m/s towards east . After 10 seconds its velocity becomes 40 m/s towards north. The average acceleration of the body is
25
A person of mass 60 kg is inside a lift of mass 940 kg and presses the button on control panel. The lift starts moving upwards with an acceleration 1.0 m/s2. If g = 10 ms$$-$$2, the tension in the supporting cable is
26
A body of mass M hits normally a rigid wall with velocity V and bounces back with the same velocity. The impulse experienced by the body is
27
The potential energy of a system increases if work is done
28
Force F on a particle moving in a straight line varies with distance d as shown in figure.

The work done on the particle during its displacement of 12 m is

The work done on the particle during its displacement of 12 m is
29
A body projected vertically from the earth reaches a height equal to earth's radius before returning to the earth. The power exerted by the gravitational force is greatest
30
A particle of mass m is released from rest and follows a parabolic path as shown. Assuming that the displacement of the mass from the origin is small, which graph correctly depicts the position of the particle as a function of time ?


31
The instantaneous angular position of a point on a rotating wheel is given by the equation $$\theta \left( t \right) = 2{t^3} - 6{t^2}$$
The torque on the wheel becomes zero at
The torque on the wheel becomes zero at
32
The moment of inertia of a thin uniform rod of mass M and length L about an axis passing through its midpoint and perpendicular to its length is $$I$$0. Its moment of inertia about an axis passing through one of its ends and perpendicular to its length is
33
During an isothermal expansion, a confined ideal gas does $$-$$ 150 J of work against its surroundings. This implies that
34
When 1 kg of ice at 0oC melts to water at 0oC, the resulting change in its entropy, taking latent heat of ice to be 80 cal/oC, is
35
Out of the following functions representing motion of a particle which represents SHM
(1) y = sin$$\omega $$t $$-$$ cos$$\omega $$t
(2) y = sin3$$\omega $$t
(3) y = 5cos$$\left( {{{3\pi } \over 4} - 3\omega t} \right)$$
(4) y = 1 + $$\omega $$t + $$\omega $$2t2
(1) y = sin$$\omega $$t $$-$$ cos$$\omega $$t
(2) y = sin3$$\omega $$t
(3) y = 5cos$$\left( {{{3\pi } \over 4} - 3\omega t} \right)$$
(4) y = 1 + $$\omega $$t + $$\omega $$2t2
36
Two waves are represented by the equations
y1 = $$a$$sin($$\omega $$t + kx + 0.57) m and
y2 = acos($$\omega $$t + kx) m, where x is in meter and t $$in$$ sec. The phase difference between them is
y1 = $$a$$sin($$\omega $$t + kx + 0.57) m and
y2 = acos($$\omega $$t + kx) m, where x is in meter and t $$in$$ sec. The phase difference between them is
37
Sound waves travel at 350 m/s through a warm air and at 3500 m/s through brass. The wavelength of a 700 Hz acoustic wave as it enters brass from warm air
38
A parallel plate capacitor has a uniform electric field E in the space between the plates. If the distance between the plates is d and area of each plate is A, the energy stored in the capacitor is
39
Four electric charges +q, +q, $$-$$ q and $$-$$ q are placed at the corners of a square of side 2L (see figure). The electric potential at point A, midway between the two charges + q and +q, is


40
A charge Q is enclosed by a Gaussian spherical surface of radius R. If the radius is doubled, then the outward electric flux will
41
A current of 2 A flows through a 2 $$\Omega $$ resistor when connected across a battery a 2 $$\Omega $$ resistor when connected across a battery. The same battery supplies a current of 0.5 A when connected across a 9 $$\Omega $$ resistor. The internal resistance of the battery is
42
If power dissipated in the 9 $$\Omega $$ resistor in the circuit shown is 36 watt, the potential difference across the 2 $$\Omega $$ resistor is
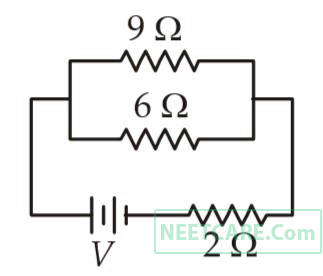

43
A uniform electric field and a uniform magnetic field are acting along the same direction in a certain region. If an electron is projected in the region such that its velocity is pointed along the direction of fields, then the electron
44
The dimensions of $${\left( {{\mu _0}{\varepsilon _0}} \right)^{ - 1/2}}$$ are