Chemistry
| List I (Molecule) | List II (Shape) |
| A $\mathrm{SF}_4$ | I. T-shaped |
| B $\mathrm{CIF}_3$ | II. Square planar |
| C $\mathrm{BrF}_5$ | III. See-saw |
| D $\mathrm{XeF}_4$ | IV. Square pyramidal |
Match the following.
| List.l (Substance) | List II (Equivalent weight) |
| A $\mathrm{Na}_2 \mathrm{CO}_3$ | I. $\frac{M}{5}$ |
| B $\mathrm{KMnO}_4 / \mathrm{H}^{+}$ | II. $\frac{M}{3}$ |
| C $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}^{+}$ | III. $\frac{M}{2}$ |
| D $\mathrm{KMnO}_4 / \mathrm{H}_2 \mathrm{O}$ | IV. $\frac{M}{6}$ |
( $M=$ Formula weight)
The correct answer is
Observe the following species.
(i) $\mathrm{NH}_3$
(ii) $\mathrm{AlCl}_3$
(iii) $\mathrm{SnCl}_4$
(iv) $\mathrm{CO}_2$
(v) $\mathrm{Ag}^{+}$
(vi) $ \mathrm{HSO}_{4}^{-} $
How many of the above species act as Lewis acids?
Consider the following reactions.
$$ \begin{array}{r} \mathrm{Cs}+\mathrm{O}_2 \text { (excess) } \rightarrow X \\ \mathrm{Na}+\mathrm{O}_2 \rightarrow Y \end{array} $$
Identify the correct Statement about $X$ and $Y$
Choose the correct statement from the following.
I. In vapour phase $\mathrm{BeCl}_2$ exists as chlorobridge dimer.
II. $\mathrm{BeSO}_4$ is readily soluble in water.
III. BeO is completely basic in nature.
IV. $\mathrm{BeCO}_3$ being unstable, is kept in the atmosphere of $\mathrm{CO}_2$.
V. $\mathrm{BeCO}_3$ is less soluble among all the carbonates of group 2 elements.
Observe the following reactions (not balanced)
$$ \begin{aligned} & \mathrm{BF}_3+\mathrm{LiAlH}_4 \xrightarrow{\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{O}} X+\mathrm{LiF}+\mathrm{AlF}_3{ }^{-} \\ & X+\mathrm{NaH} \longrightarrow Y \end{aligned} $$
The incorrect statement about $Y$ is
$\mathbf{Assertion (A)}$ Silicones are used for water proofing of fabrics.
$\mathbf{Reason (R)}$ The repeating unit in silicones is

The correct answer is
The correct IUPAC name of the structure given below is

The major product ' $Y^{\prime}$ in the given sequence of reactions is
$$ \mathrm{C}_3 \mathrm{H}_7 \mathrm{OH} \xrightarrow[443 \mathrm{~K}]{\text { Conc. } \mathrm{H}_2 \mathrm{SO}_4} X \xrightarrow[\left(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CO}\right)_2 \mathrm{O}_2]{\mathrm{HBr}} Y $$
$\mathrm{NH}_2 \mathrm{CONH}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow[\mathrm{X}] \rightleftharpoons 2 \mathrm{NH}_3+\mathrm{H}_2 \mathrm{O}+[\mathrm{Y}]$
The hybridisation of carbon in $X$ and $Y$ respectively are
Identify the structure of the polymer 'P' formed in the given reaction
Caprolactam $\xrightarrow[\mathrm{H}_2 \mathrm{O}]{533-543 \mathrm{~K}} P$

Conversion of $X$ to $Y$ in the above reaction is
$$ \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \xrightarrow[443 \mathrm{~K}]{\text { Conc. } \mathrm{H}_2 \mathrm{SO}_4} X \xrightarrow[\text { (ii) } \mathrm{Zn} / \mathrm{H}_2 \mathrm{O}\,(2 Moless)]{\text { (i) } \mathrm{O}_3} Y \xrightarrow[\text { (i) } \mathrm{H}^{+}]{\text {(i) Conc. } \mathrm{NaOH}} \mathrm{Z} $$
$Z$ is a mixture of alcohol and acid. Reaction of conversion of $Y$ to $Z$ is
Arrange the following in the increasing order of pKa values

What is ' $C$ ' in the following reaction sequence?

Identify the products $R$ and $S$ in the reaction sequence given
$\left(\mathrm{CH}_3\right)_3 \mathrm{COH} \xrightarrow{\mathrm{Na}} P \xrightarrow{\mathrm{CH}_3 \mathrm{Br}} Q \xrightarrow[\Delta]{\mathrm{HI}} R+S$
In the given reaction sequence sequence, $Z$ is

Mathematics
If $A=\left[\begin{array}{lll}x & y & y \\ y & x & y \\ y & y & x\end{array}\right]$ is a matrix such that $5 A^{-1}=\left[\begin{array}{ccc}-3 & 2 & 2 \\ 2 & -3 & 2 \\ 2 & 2 & -3\end{array}\right]$, then $A^2-4 A=$
If $A=\left[\begin{array}{lll}9 & 3 & 0 \\ 1 & 5 & 8 \\ 7 & 6 & 2\end{array}\right]$ and $A A^T-A^2=\left[\begin{array}{lll}a_{11} & a_{12} & a_{13} \\ a_{21} & a_{22} & a_{23} \\ a_{31} & a_{32} & a_{33}\end{array}\right]$, then $\sum\limits_{\substack{1 \leq i \leq 3 \\ 1 \leq j \leq 3}} a_{i j}=$
If $a \neq b \neq c, \Delta_1=\left[\begin{array}{lll}1 & a^2 & b c \\ 1 & b^2 & c a \\ 1 & c^2 & a b\end{array}\right]$, $\Delta_2=\left[\begin{array}{ccc}1 & 1 & 1 \\ a^2 & b^2 & c^2 \\ a^3 & b^3 & c^3\end{array}\right]$ and $\frac{\Delta_1}{\Delta_2}=\frac{6}{11}$, then $11(a+b+c)=$
The system of equations $x+3 y+7=0$, $3 x+10 y-3 z+18=0$ and $3 y-9 z+2=0$ has
| $X=x$ | 2 | 3 | 5 | 9 |
| $P(X=x)$ | $k$ | $2 k$ | $3 k^2$ | $k$ |
Physics
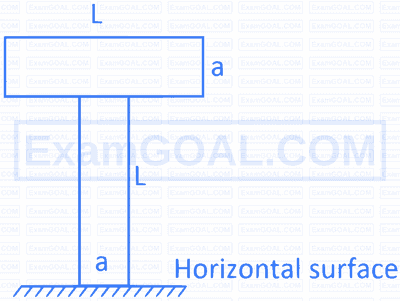
A block of mass 2 kg is tied to one end of a 2 m long metal wire of $1.0 \mathrm{~mm}^2$ area of cross-section and rotated in a vertical circle such that the tension in the wire is zero at the highest point. If the maximum elongation in the wire is 2 mm , the Young's modulus of the metal is
(Acceleration due to gravity $=10 \mathrm{~ms}^{-2}$ )
A conductor of length 1.5 m and area of cross-section $3 \times 10^{-5} \mathrm{~m}^2$ has electrical resistance of $15 \Omega$.
The current density in the conductor for an electric field of $21 \mathrm{Vm}^{-1}$ is