Chemistry
1. For the reaction,
$$A(g)+B(g) \rightleftharpoons C(g)+D(g) ; \Delta H=Q \mathrm{~kJ}$$
The equilibrium constant cannot b 2. An organic compound $$X$$ on treatment with PCC in dichloromethane gives the compound $$Y$$. Compound $$Y$$ reacts with 3. A compound $$'A' \left(\mathrm{C}_7 \mathrm{H}_8 \mathrm{O}\right)$$ is insoluble in $$\mathrm{NaHCO}_3$$ solution but d 4. In set of reactions, identify $$D$$
$$\mathrm{CH}_3 \mathrm{COOH} \xrightarrow{\mathrm{SOCl}_2} A \xrightarrow[\text { A 5. $$K_a$$ values for acids $$\mathrm{H}_2 \mathrm{SO}_3, \mathrm{HNO}_2, \mathrm{CH}_3 \mathrm{COOH}$$ and $$\mathrm{HCN}$ 6.
$$A, B$$ and $$C$$ respectively are 7. The reagent which can do the conversion $$\mathrm{CH}_3 \mathrm{COOH} \longrightarrow \mathrm{CH}_3-\mathrm{CH}_2-\mathr 8. $$\begin{aligned}
& \mathrm{CH}_3 \mathrm{CHO} \xrightarrow[\text { (ii) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \ma 9. Which of the following is not true for oxidation? 10. Which is the most suitable reagent for the following conversion?
11. $$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Cl} \xrightarrow{\text { Alc. } \mathrm{NH}_3} A \xrightarrow{2 \mathr 12. The method by which aniline cannot be prepared is 13. Permanent hardness cannot be removed by 14. A hydrocarbon $$A\left(\mathrm{C}_4 \mathrm{H}_8\right)$$ on reaction with $$\mathrm{HCl}$$ gives a compound $$\mathrm{B 15. RNA and DNA are chiral molecules, their chirality is due to the presence of 16. The property of the alkaline earth metals that increases with their atomic number is 17. Primary structure in a nucleic acid contain 3 bases as GATGC ... The chain which is complementary to this chain is 18. In the detection of II group acid radical, the salt containing chloride is treated with concentrated sulphuric acid, the 19. The number of six membered and five membered rings in Buckminster fullerene respectively is 20. In chrysoberyl, a compound containing beryllium, aluminium and oxygen, oxide ions form cubic close packed structure. Alu 21. The correct statement regarding defects in solid is 22. A metal crystallises in bcc lattice with unit cell edge length of $$300 \mathrm{~pm}$$ and density $$615 \mathrm{~g~cm}^ 23. Henry's law constant for the solubility of $$\mathrm{N}_2$$ gas in water at $$298 \mathrm{~K}$$ is $$1.0 \times 10^5 \ma 24. A pure compound contains $$2.4 \mathrm{~g}$$ of $$\mathrm{C}, 1.2 \times 10^{23}$$ atoms of $$\mathrm{H}, 0.2$$ moles of 25. Choose the correct statement. 26. The $$K_{\mathrm{H}}$$ value ($$\mathrm{K}$$ bar) of argon (I), carbondioxide (II), formaldehyde (III) and methane (IV) 27. The vapour pressure of pure liquids $$A$$ and $$B$$ are 450 and $$700 \mathrm{~mm}$$ of $$\mathrm{Hg}$$ at $$350 \mathrm 28. Consider the following electrodes
$$\begin{aligned}
& P=\mathrm{Zn}^{2+}(0.0001 \mathrm{M}) / \mathrm{Zn}, Q=\mathrm{Zn} 29. The number of angular and radial nodes in $$3 p$$ orbital respectively are 30. The resistance of $$0.01 \mathrm{~m} \mathrm{~KCl}$$ solution at $$298 \mathrm{~K}$$ is $$1500 \Omega$$. If the conducti 31. $$\mathrm{H}_2(g)+2 \mathrm{AgCl}(s) \rightleftharpoons 2 \mathrm{Ag}(s)+2 \mathrm{HCl}(a q)$$
$$E_{\text {cell }}^{\cir 32. For a reaction, $$A+2 B \rightarrow$$ Products, when concentration of $$B$$ alone is increased half-life remains the sam 33. The third ionisation enthalpy is highest in 34. If the rate constant for a first order reaction is $$k$$, the time $$(t)$$ required for the completion of $$99 \%$$ of t 35. The rate of a gaseous reaction is given by the expression $$k[A][B]^2$$. If the volume of vessel is reduced to one half 36. The correct IUPAC name of 37. Higher order $$(>3)$$ reactions are rare due to 38. Arrange benzene, $$n$$-hexane and ethyne in decreasing order of their acidic behaviour. 39. A colloidal solution is subjected to an electric field than colloidal particles more towards anode. The amount of electr 40. Which of the following is an incorrect statement? 41. Zeta potential is 42. Which of the following compound on heating gives $$\mathrm{N}_2 \mathrm{O}$$ ? 43. Which of the following property is true for the given sequence?
$$\mathrm{NH}_3>\mathrm{PH}_3>\mathrm{AsH}_3>\mathrm{SbH 44. The correct order of boiling point in the following compounds is 45. $$\mathrm{XeF}_6$$ on partial hydrolysis gives a compound $$X$$, which has square pyramidal geometry '$$X$$' is 46. A colourless, neutral, paramagnetic oxide of nitrogen '$$P$$' on oxidation gives reddish brown gas $$Q$$. $$Q$$ on cooli 47. Which of the following does not represent property stated against it? 48. Which one of the following is correct for all elements from Sc to Cu? 49. When the absolute temperature of ideal gas is doubled and pressure is halved, the volume of gas 50. Which of the following pairs has both the ions coloured in aqueous solution? [Atomic numbers of
$$\mathrm{Sc}=21, \mathr 51. For the crystal field splitting in octahedral complexes, 52. Peroxide effect is observed with the addition of $$\mathrm{HBr}$$ but not with the addition of HI to unsymmetrical alken 53. The IUPAC name of $$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{CO}_3\right)\right] \mathrm{Cl}$$ is
54. Homoleptic complexes among the following are
(A) $$\mathrm{K}_3\left[\mathrm{Al}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3 55. The correct order for wavelengths of light absorbed in the complex ions $$\left[\mathrm{CoCl}\left(\mathrm{NH}_3\right)_ 56.
The compound A (major product) is 57. Bond enthalpies of $$A_2, B_2$$ and $$A B$$ are in the ratio $$2: 1: 2$$. If bond enthalpy of formation of $$A B$$ is $$ 58. The order of reactivity of the compounds $$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}, \mathrm{C}_6 \mathrm{H}_ 59. The major product of the following reaction is
$$\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2-\mathrm{OH} \xrightarrow[\text 60.
The product '$$A$$' gives white precipitate when treated with bromine water. The product '$$B$$' is treated with barium
Mathematics
1. The equation of the line joining the points $$(-3,4,11)$$ and $$(1,-2,7)$$ is 2. The angle between the lines whose direction cosines are $$\left(\frac{\sqrt{3}}{4}, \frac{1}{4}, \frac{\sqrt{3}}{2}\righ 3. If a plane meets the coordinate axes at $$A, B$$ and $$C$$ in such a way that the centroid of $$\triangle A B C$$ is at 4. The area of the quadrilateral $$A B C D$$ when $$A(0,4,1), B(2,3,-1), C(4,5,0)$$ and $$D(2,6,2)$$ is equal to 5. The shaded region is the solution set of the inequalities
6. Given that, $$A$$ and $$B$$ are two events such that $$P(B)=\frac{3}{5}, P\left(\frac{A}{B}\right)=\frac{1}{2}$$ and $$P 7. If $$A, B$$ and $$C$$ are three independent events such that $$P(A)=P(B)=P(C)=P$$, then $$P$$ (at least two of $$A, B$$ 8. Two dice are thrown. If it is known that the sum of numbers on the dice was less than 6 the probability of getting a sum 9. A car manufacturing factory has two plants $$X$$ and $$Y$$. Plant $$X$$ manufactures $$70 \%$$ of cars and plant $$Y$$ m 10. In a certain two $$65 \%$$ families own cell phones, 15000 families own scooter and $$15 \%$$ families own both. Taking 11. $$A$$ and $$B$$ are non-singleton sets and $$n(A \times B)=35$$. If $$B \subset A$$, then $${ }^{n(A)} C_{n(B)}$$ is equ 12. Domain of $$f(x)=\frac{x}{1-|x|}$$ is 13. The value of $$\cos 1200^{\circ}+\tan 1485^{\circ}$$ is 14. The value of $$\tan 1^{\circ} \tan 2^{\circ} \tan 3^{\circ} \ldots \tan 89^{\circ}$$ is 15. If $$\left(\frac{1+i}{1-i}\right)^x=1$$, then 16. The cost and revenue functions of a product are given by $$c(x)=20 x+4000$$ and $$R(x)=60 x+2000$$ respectively, where $ 17. A student has to answer 10 questions, choosing at least 4 from each of the parts $$A$$ and $$B$$. If there are 6 questio 18. If the middle term of the AP is 300, then the sum of its first 51 terms is 19. The equation of straight line which passes through the point $$\left(a \cos ^3 \theta, a \sin ^3 \theta\right)$$ and per 20. The mid points of the sides of triangle are $$(1,5,-1)(0,4,-2)$$ and $$(2,3,4)$$ then centroid of the triangle 21. Consider the following statements
Statement 1 : $$\lim _\limits{x \rightarrow 1} \frac{a x^2+b x+c}{x^2+b x+a}$$ is 1
(w 22. If $$a$$ and $$b$$ are fixed non-zero constants, then the derivative of $$\frac{a}{x^4}-\frac{b}{x^2}+\cos x$$ is $$m a+ 23. The standard deviation of the numbers $$31,32,33 \ldots 46,47$$ is 24. If $$P(A)=0.59, P(B)=0.30$$ and $$P(A \cap B)=0.21$$ then $$P\left(A^{\prime} \cap B^{\prime}\right)$$ is equal to 25. $$f: R \rightarrow R$$ defined by $$f(x)$$ is equal to $$\left\{\begin{array}{l}2 x, x> 3 \\ x^2, 1 26. Let $$A=\{x: x \in R, x$$ is not a positive integer) Define $$f: A \rightarrow R$$ as $$f(x)=\frac{2 x}{x-1}$$, then $$f 27. The function $$f(x)=\sqrt{3} \sin 2 x-\cos 2 x+4$$ is one-one in the interval 28. Domain of the function
$$f(x)=\frac{1}{\sqrt{\left[x^2\right]-[x]-6}},$$
where $$[x]$$ is greatest integer $$\leq x$$ is 29. $$\cos \left[\cot ^{-1}(-\sqrt{3})+\frac{\pi}{6}\right]$$ is equal to 30. $$\tan ^{-1}\left[\frac{1}{\sqrt{3}} \sin \frac{5 \pi}{2}\right] \sin ^{-1}\left[\cos \left(\sin ^{-} \frac{\sqrt{3}}{2} 31. If $$A=\left[\begin{array}{ccc}1 & -2 & 1 \\ 2 & 1 & 3\end{array}\right]$$
$$ B=\left[\begin{array}{ll}2 & 1 \\ 3 & 2 \\ 32. Let $$M$$ be $$2 \times 2$$ symmetric matrix with integer entries, then $$M$$ is invertible if 33. If $$A$$ and $$B$$ are matrices of order 3 and $$|A|=5,|B|=3$$, then $$|3 A B|$$ is 34. If $$A$$ and $$B$$ are invertible matrices then which of the following is not correct? 35. If $$f(x)=\left|\begin{array}{ccc}\cos x & 1 & 0 \\ 0 & 2 \cos x & 3 \\ 0 & 1 & 2 \cos x\end{array}\right|$$, then $$\li 36. If $$x^3-2 x^2-9 x+18=0$$ and $$A=\left|\begin{array}{lll}1 & 2 & 3 \\ 4 & x & 6 \\ 7 & 8 & 9\end{array}\right|$$ then t 37. At $$x=1$$, the function
$$f(x)=\left\{\begin{array}{cc}
x^3-1, & 1 38. If $$y=\left(\cos x^2\right)^2$$, then $$\frac{d y}{d x}$$ is equal to 39. For constant $$a, \frac{d}{d x}\left(x^x+x^a+a^x+a^a\right)$$ is 40. Consider the following statements
Statement 1 : If $$y=\log _{10} x+\log _e x$$, then $$\frac{d y}{d x}=\frac{\log _{10} 41. If the parametric equation of curve is given by $$x=\cos \theta+\log \tan \frac{\theta}{2}$$ and $$y=\sin \theta$$, then 42. If $$y=(x-1)^2(x-2)^3(x-3)^5$$, then $$\frac{d y}{d x}$$ at $$x=4$$ is equal to 43. A particle starts form rest and its angular displacement (in radians) is given by $$\theta=\frac{t^2}{20}+\frac{t}{5}$$. 44. If the parabola $$y=\alpha x^2-6 x+\beta$$ passes through the point $$(0,2)$$ and has its tangent at $$x=\frac{3}{2}$$ p 45. The function $$f(x)=x^2-2 x$$ is strictly decreasing in the interval 46. The maximum slope of the curve $$y=-x^3+3 x^2+2 x-27$$ is 47. $$\int \frac{x^3 \sin \left(\tan ^{-1}\left(x^4\right)\right)}{1+x^8} d x$$ is equal to 48. The value of $$\int \frac{x^2 d x}{\sqrt{x^6+a^6}}$$ is equal to 49. The value of $$\int \frac{x e^x d x}{(1+x)^2}$$ is equal to 50. The value of $$\int e^x\left[\frac{1+\sin x}{1+\cos x}\right] d x$$ is equal to 51. If $$I_n=\int_0^{\frac{\pi}{4}} \tan ^n x d x$$, where $$n$$ is positive integer, then $$I_{10}+I_8$$ is equal to 52. The value of $$\int_0^{4042} \frac{\sqrt{x} d x}{\sqrt{x}+\sqrt{4042-x}}$$ is equal to 53. The area of the region bounded by $$y=-\sqrt{16-x^2}$$ and $$X$$-axis is 54. If the area of the ellipse is $$\frac{x^2}{25}+\frac{y^2}{\lambda^2}=1$$ is $$20 \pi$$ sq units, then $$\lambda$$ is 55. Solution of differential equating $$x d y-y d x=0$$ represents 56. The number of solutions of $$\frac{d y}{d x}=\frac{y+1}{x-1}$$, when $$y(\mathrm{l})=2$$ is 57. A vector a makes equal acute angles on the coordinate axis. Then the projection of vector $$\mathbf{b}=5 \hat{\mathbf{i} 58. The diagonals of a parallelogram are the vectors $$3 \hat{\mathbf{i}}+6 \hat{\mathbf{j}}-2 \hat{\mathbf{k}}$$. and $$-\h 59. If $$\mathbf{a} \cdot \mathbf{b}=0$$ and $$\mathbf{a}+\mathbf{b}$$ makes an angle $$60^{\circ}$$ with $$a$$, then 60. If the area of the parallelogram with $$\mathbf{a}$$ and $$\mathbf{b}$$ as two adjacent sides is 15 sq units, then the a
Physics
1. The physical quantity which is measure in the unit of wb $$\mathrm{A}^{-1}$$ is 2. What will be the reading in the voltmeter and ammeter of the circuit shown?
3. LC-oscillations are similar and analogous to the mechanical oscillations of a block attached to a spring. The electrical 4. In an oscillating $$L C$$-circuit, $$L=3 \mathrm{mH}$$ and $$C=2.7 \mu \mathrm{F}$$. At $$t=0$$, the charge on the capac 5. Suppose that the electric field amplitude of electromagnetic wave is $$E_0=120 \mathrm{~NC}^{-1}$$ and its frequency $$f 6. The source of electromagnetic wave can be a charge 7. In refraction, light waves are bent on passing from one medium to second medium because, in the second medium 8. If the refractive index from air to glass is $$\frac{3}{2}$$ and that from air to water is $$\frac{4}{3}$$, then the rat 9. Two thin biconvex lenses have focal lengths $$f_1$$ and $$f_2$$. A third thin biconcave lens has focal length of $$f_3$$ 10. The size of the image of an object, which is at infinity, as formed by a convex lens of focal length $$30 \mathrm{~cm}$$ 11. A slit of width $$a$$ is illuminated by red light of wavelength $$6500 \mathop A\limits^o$$. If the first diffraction mi 12. Which of the following statements are correct with reference to single slit diffraction pattern?
(I) Fringes are of uneq 13. In the Young's double slit experiment a monochromatic source of wavelength $$\lambda$$ is used. The intensity of light p 14. The work-function of a metal is 1 eV. Light of wavelength $$3000 \mathop A\limits^o$$ is incident on this metal surface. 15. A proton moving with a momentum $$p_1$$ has a kinetic energy $$1 / 8$$th of its rest mass-energy. Another light photon h 16. According to Einstein's photoelectric equation to the graph between kinetic energy of photoelectrons ejected and the fre 17. Energy of an electron in the second orbit of hydrogen atom is $$E_2$$. The energy of electron in the third orbit of $$\m 18. The figure shows standing de-Broglie waves due to the revolution of electron in a certain orbit of hydrogen atom. Then, 19. An electron in an excited state of $$\mathrm{Li}^{2+}$$ ion has angular momentum $$\frac{3 h}{2 \pi}$$. The de-Broglie w 20. Which graph in the following diagram correctly represents the potential energy of a pair of nucleons as a function of th 21. In a nuclear reactor heavy nuclei is not used as moderators because 22. The circuit given represents which of the logic operations?
23. Identify the incorrect statement. 24. Three photodiodes $$D_1, D_2$$ and $$D_3$$ are made of semiconductors having band gaps of $$2.5 \mathrm{~eV}, 2 \mathrm{ 25. For a body moving along a straight line, the following $$v$$-$$t$$ graph is obtained.
According to the graph, the displ 26. A particle starts from rest. Its acceleration $$a$$ versus time $$t$$ is shown in the figure. The maximum speed of the p 27. The maximum range of a gun on horizontal plane is $$16 \mathrm{~km}$$. If $$\mathrm{g}=10 \mathrm{~ms}^{-2}$$, then muzz 28. The trajectory of projectile is 29. For a projectile motion, the angle between the velocity and acceleration is minimum and acute at 30. A particle starts from the origin at $$t=0$$ with a velocity of $$10 \hat{\mathbf{j}} \mathrm{ms}^{-1}$$ and move in the 31. A coin placed on a rotating turn table just slips if it is placed at a distance of $$4 \mathrm{~cm}$$ from the centre. I 32. A $$1 \mathrm{~kg}$$ ball moving at $$12 \mathrm{~ms}^{-1}$$ collides with a $$2 \mathrm{~kg}$$ ball moving in opposite 33. A ball hits the floor and rebounds after an inelastic collision. In this case 34. In figure $$E$$ and $$v_{\mathrm{cm}}$$ represent the total energy and speed of centre of mass of an object of mass $$1 35. Two bodies of masses $$8 \mathrm{~kg}$$ are placed at the vertices $$A$$ and $$B$$ of an equilateral triangle $$A B C$$. 36. Two capillary tubes $$P$$ and $$Q$$ are dipped vertically in water. The height of water level in capillary tube $$P$$ is 37. Which of the following curves represent the variation of coefficient of volume expansion of an ideal gas at constant pre 38. A number of Carnot engines are operated at identical cold reservoir temperatures $$(T_L)$$. However, their hot reservoir 39. A gas mixture contains monoatomic and diatomic molecules of 2 moles each. The mixture has a total internal energy of (sy 40. A pendulum oscillates simple harmonically and only if
I. the sizer of the bob of pendulum is negligible in comparison wi 41. To propagate both longitudinal and transverse waves, a material must have 42. A copper rod $$A B$$ of length $$l$$ is rotated about end $$A$$ with a constant angular velocity $$\omega$$. The electri 43. Electric field due to infinite, straight uniformly charged wire varies with distance $$r$$ as 44. A $$2 \mathrm{~g}$$ object, located in a region of uniform electric field $$\mathrm{E}=\left(300 \mathrm{NC}^{-1}\right) 45. If a slab of insulating material (conceptual). $$4 \times 10^{-3} \mathrm{~m}$$ thick is introduced between the plates o 46. Eight drops of mercury of equal radii combine to form a big drop. The capacitance of a bigger drop as compared to each s 47. Which of the following statements is false in the case of polar molecules? 48. An electrician requires a capacitance of $$6 \mu \mathrm{F}$$ in a circuit across a potential difference of $$1.5 \mathr 49. In figure, charge on the capacitor is plotted against potential difference across the capacitor. The capacitance and ene 50. A wire of resistance $$3 \Omega$$ is stretched to twice its original length. The resistance of the new wire will be 51. In the given arrangement of experiment on meter bridge, if $$A D$$ corresponding to null deflection of the galvanometer 52. A copper wire of length $$1 \mathrm{~m}$$ and uniform cross-sectional area $$5 \times 10^{-7} \mathrm{~m}^2$$ carries a 53. Consider an electrical conductor connected across a potential difference $$V$$. Let $$\Delta q$$ be a small charge movin 54. A strong magnetic field is applied on a stationary electron. Then, the electron 55. Two parallel wires in free space are $$10 \mathrm{~cm}$$ apart and each carries a current of $$10 \mathrm{~A}$$ in the s 56. A toroid with thick windings of $$N$$ turns has inner and outer radii $$R_1$$ and $$R_2$$, respectively. If it carries c 57. A tightly wound long solenoid has $n$ turns per unit length, a radius $$r$$ and carries a current $$I$$. A particle havi 58. Earth's magnetic field always has a horizontal component except at 59. Which of the field pattern given below is valid for electric field as well as for magnetic field? 60. The current following through an inductance coil of self-inductance $$6 \mathrm{~mH}$$ at different time instants is as
1
KCET 2021
MCQ (Single Correct Answer)
+1
-0
According to Einstein's photoelectric equation to the graph between kinetic energy of photoelectrons ejected and the frequency of incident radiation is
A
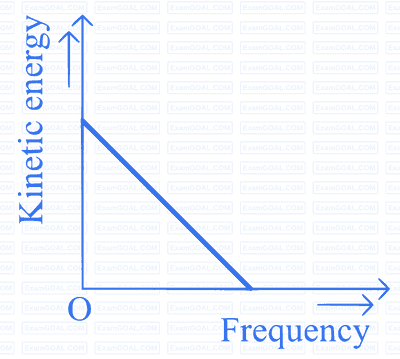
B

C
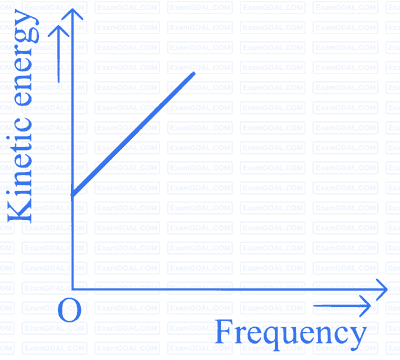
D

2
KCET 2021
MCQ (Single Correct Answer)
+1
-0
Energy of an electron in the second orbit of hydrogen atom is $$E_2$$. The energy of electron in the third orbit of $$\mathrm{He}^{+}$$ will be
A
$$\frac{9}{16} E_2$$
B
$$\frac{16}{9} E_2$$
C
$$\frac{3}{16} E_2$$
D
$$\frac{16}{3} E_2$$
3
KCET 2021
MCQ (Single Correct Answer)
+1
-0
The figure shows standing de-Broglie waves due to the revolution of electron in a certain orbit of hydrogen atom. Then, the expression for the orbit radius is (All notations have their usual meanings)

A
$$\frac{h^2 \varepsilon_0}{\pi m e^2}$$
B
$$\frac{4 h^2 \varepsilon_0}{\pi m e^2}$$
C
$$\frac{9 h^2 \varepsilon_0}{\pi m e^2}$$
D
$$\frac{16 h^2 \varepsilon_0}{\pi m e^2}$$
4
KCET 2021
MCQ (Single Correct Answer)
+1
-0
An electron in an excited state of $$\mathrm{Li}^{2+}$$ ion has angular momentum $$\frac{3 h}{2 \pi}$$. The de-Broglie wavelength of electron in this state is $$p \pi a_0$$ (where, $$a_0=$$ Bohr radius). The value of $$p$$ is
A
3
B
2
C
1
D
4