Chemistry
| $$ \text { List-I } $$ |
$$ \text { List-II } $$ |
||
|---|---|---|---|
| A | $$ \text { Ionisation enthalpy } $$ |
I | $$ \mathrm{P}<\mathrm{Si}<\mathrm{Mg}<\mathrm{Na} $$ |
| B | $$ \text { Metallic character } $$ |
II | $$ \mathrm{I}<\mathrm{N}<\mathrm{O}<\mathrm{F} $$ |
| C | $$ \text { Electron gain enthalpy } $$ |
III | $$ \mathrm{B}<\mathrm{Be}<\mathrm{C}<\mathrm{O}<\mathrm{N} $$ |
| D | $$ \text { Electronegativity } $$ |
IV | $$ \mathrm{l}<\mathrm{Br}<\mathrm{F}<\mathrm{Cl} $$ |
$$ \text { Observe the following structure, } $$

$$ \text { The formal charges on the atoms 1,2,3 respectively are } $$
Two statement are given below.
Statement I : The ratio of the molar volume of a gas to that of an ideal gas at constant temperature and pressure is called the compressibility factor.
Statement II : The rms velocity of a gas is directly proportional to square root of $T(\mathrm{~K})$.
The correct answer is
Given below are two statements.
Statement I : In the decomposition of potassium chlorate Cl is reduced.
Statement II : Reaction of Na with $\mathrm{O}_2$ to form $\mathrm{Na}_2 \mathrm{O}$ is a redox reaction.
The correct answer is
Observe the following reaction,
$$ 2 A_2(g)+B_2(g) \xrightarrow{T(\mathrm{~K})} 2 A_2 B(g)+600 \mathrm{~kJ} $$
The standard enthalpy of formation $\left(\Delta_f H^{\ominus}\right)$ of $A_2 B(g)$ is

$$ \text { Match the following. } $$
| List-I F-ion concentration in drinking water) |
List-II (Effects on humans) |
||
|---|---|---|---|
| A | $$ \text { <1 ppm } $$ |
I | $$ \text { Harmful to bones } $$ |
| B | $$ >2 \mathrm{ppm} $$ |
II | $$ \text { Tooth decay } $$ |
| C | $$ >10 \mathrm{ppm} $$ |
III | $$ \text { Brown mottling of teeth } $$ |
The number of nucleophiles in the following list is
$$ \mathrm{CH}_3 \mathrm{NH}_2, \mathrm{CH}_3 \mathrm{CHO}, \mathrm{C}_2 \mathrm{H}_4, \mathrm{CH}_3 \mathrm{SH} $$
An alkene $X\left(\mathrm{C}_4 \mathrm{H}_8\right)$ on reaction with HBr gave $Y\left(\mathrm{C}_4 \mathrm{H}_9 \mathrm{Br}\right)$. Reaction of $Y$ with benzene in the presence of anhydrous $\mathrm{AlCl}_3$ gave Z which is resistant to oxidation with $\mathrm{KMnO}_4-\mathrm{KOH}$. What are $X, Y, Z$ respectively?
A solid compound is formed by atoms of $A$ (cations), $B$ (cations) and O (anions). Atoms of O form hcp lattice. Atoms of $A$ occupy $25 \%$ of tetrahedral holes and atoms of $B$ occupy $50 \%$ octahedral holes. What is the molecular formula of solid?
The density of nitric acid solution is $1.5 \mathrm{~g} \mathrm{~mL}^{-1}$. Its weight percentage is 68 . What is the approximate concentration (in $\mathrm{mol} \mathrm{L}^{-1}$ ) of nitric acid ?
$$ (\mathrm{N}=14 \mathrm{u} ; \mathrm{O}=16 \mathrm{u} ; \mathrm{H}=1 \mathrm{u}) $$
The osmotic pressure of sea water is 1.05 atm . Four experiments were carried as shown in table. In which of the following experiments, pure water can be obtained in part-II of vessel.
 $$
\begin{aligned}
&\text { Table }\\
&\begin{array}{ccc}
\hline \begin{array}{c}
\text { Expt. } \\
\text { No }
\end{array} & \begin{array}{c}
\text { Pressure applied in } \\
\text { part-1 of vessel }
\end{array} & \begin{array}{c}
\text { Pressure applied in } \\
\text { part-II of vessel }
\end{array} \\
\hline \text { I. } & 10 \mathrm{~atm} & - \\
\hline \text { II. } & - & 10 \mathrm{~atm} \\
\hline \text { III. } & 15 \mathrm{~atm} & - \\
\hline \text { IV. } & - & 15 \mathrm{~atm}
\end{array}
\end{aligned}
$$
$$
\begin{aligned}
&\text { Table }\\
&\begin{array}{ccc}
\hline \begin{array}{c}
\text { Expt. } \\
\text { No }
\end{array} & \begin{array}{c}
\text { Pressure applied in } \\
\text { part-1 of vessel }
\end{array} & \begin{array}{c}
\text { Pressure applied in } \\
\text { part-II of vessel }
\end{array} \\
\hline \text { I. } & 10 \mathrm{~atm} & - \\
\hline \text { II. } & - & 10 \mathrm{~atm} \\
\hline \text { III. } & 15 \mathrm{~atm} & - \\
\hline \text { IV. } & - & 15 \mathrm{~atm}
\end{array}
\end{aligned}
$$Aqueous $\mathrm{CuSO}_4$ solution was electrolysed by passing 2 amp of current for 10 min . What is the weight (in g) of copper deposited at cathode ?
$$ \left(\mathrm{Cu}=63 \mathrm{u} ; F=96500 \mathrm{C} \mathrm{~mol}^{-1}\right) $$
For a first order reaction the concentration of reactant was reduced from $0.03 \mathrm{molL}^{-1}$ to $0.02 \mathrm{molL}^{-1}$ in 25 min . What is its rate (in $\mathrm{molL}^{-1} \mathrm{~s}^{-1}$ )?
' $X$ ' is a protecting colloid. The following data is obtained for preventing the coagulation of 10 mL of gold sol to which 1 mL of $10 \% \mathrm{NaCl}$ is added. What is the gold number of ' $X$ '?
$$ \begin{array}{ccl} \hline \text { Expt No. } & \begin{array}{c} \text { Weight of } X \text { (in mg) } \\ \text { added to gold sol } \end{array} & \text { Coagulation } \\ \hline 1 & 24 & \text { Not prevented } \\ \hline 2 & 23 & \text { Not prevented } \\ \hline 3 & 26 & \text { Prevented } \\ \hline 4 & 27 & \text { Prevented } \\ \hline 5 & 25 & \text { Prevented } \\ \hline \end{array} $$
The reactions which occur in blast furnace at $500-800 \mathrm{~K}$ during extraction of iron from haematite are
i. $3 \mathrm{Fe}_2 \mathrm{O}_3+\mathrm{CO} \longrightarrow 2 \mathrm{Fe}_3 \mathrm{O}_4+\mathrm{CO}_2$
ii. $\mathrm{Fe}_2 \mathrm{O}_3+3 \mathrm{C} \longrightarrow 2 \mathrm{Fe}+3 \mathrm{CO}$
iii. $\mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{CO} \longrightarrow \mathrm{Fe}+4 \mathrm{CO}_2$
iv. $\mathrm{Fe}_2 \mathrm{O}_3+\mathrm{CO} \longrightarrow 2 \mathrm{FeO}+\mathrm{CO}_2$
Which of the following reactions give phosphine?
i. Reaction of calcium phosphide with water
ii. Heating white phosphorous with concentrated NaOH solution in inert atmosphere
iii. Heating red phosphorous with alkali
$$ \text { Which of the following is an example for fibre? } $$
An alkene $X\left(\mathrm{C}_4 \mathrm{H}_8\right)$ does not exhibit cis-trans is memerisn Reaction of $X$ with $\mathrm{Br}_2$ in the presence of UV light gane $Y$. What is $Y$ ?
$$ \text { Consider the reactions, } $$
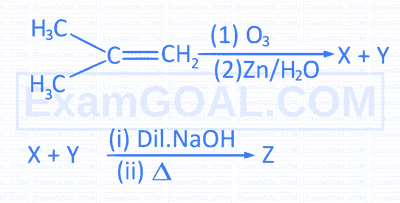
 $Y$ and $Z$ respectively are
$Y$ and $Z$ respectively are$$ \text { The incorrect statement about ' } B \text { ' is } $$

$$ \text { The incorrect statement about } B \text { is } $$
$$ \text { Benzamide } \xrightarrow{\mathrm{Br}_2 / \mathrm{NaOH}} X \xrightarrow[\text { Alc. } \mathrm{KOH}]{\mathrm{CHCl}_3} Y $$
The conversion of $X$ to $Y$ is
Mathematics
Let $a > 1$ and $0 < \mathrm{b} < 1$. If $f: R \rightarrow[0,1]$ is defined by $f(x)=\left\{\begin{array}{ll}a^x, & -\infty < x < 0 \\ b^x, & 0 \leq x < \infty\end{array}\right.$, then $f(x)$ is
| List I | List II | ||
|---|---|---|---|
| (A) | $$ \sum \cot A $$ |
(i) | $$ (a+b+c)^2 \frac{1}{4 \Delta} $$ |
| (B) | $$ \sum \cot \frac{A}{2} $$ |
(ii) | $$ \left(a^2+b^2+c^2\right) \frac{1}{4 \Delta} $$ |
| (C) | If $\tan A: \tan B: \tan C=1: 2: 3$, then $\sin A: \sin B: \sin C=$ | (iii) | $$ 8: 6: 5 $$ |
| (D) | $$ \begin{aligned} &\text { If } \cot \frac{A}{2}: \cot \frac{B}{2}: \cot \frac{C}{2}=3: 7: 9\\ &\text { then } a: b: c= \end{aligned} $$ |
(iv) | $$ 12: 5: 13 $$ |
| (v) | $$ \sqrt{5}: 2 \sqrt{2}: 3 $$ |
||
| (vi) | $$ 4 \Delta $$ |
||
S is the sample space and $A, B$ are two events of a random experiment. Match the items of List $A$ with the items of List B
| $$ \text { List A } $$ |
$$ \text { List B } $$ |
||
|---|---|---|---|
| I | $A, B$ are mutually exclusive events | a. | $$ P(A \cap B)=P(B)-P(\bar{A}) $$ |
| II | $$ A, B \text { are independent events } $$ |
b. | $$ P(A) \leq P(B) $$ |
| III | $$ A \cap B=A $$ |
c. | $$ P\left(\frac{\bar{A}}{B}\right)=1-P(A) $$ |
| IV | $$ A \cup B=S $$ |
d. | $$ P(A \cup B)=P(A)+P(B) $$ |
| e. | $$ P(A)+P(B)=2 $$ |
||
| X = x | -2 | -1 | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|
| P(X = x) | $$ \frac{1}{10} $$ |
$$ K+\frac{2}{10} $$ |
$$ K+\frac{3}{10} $$ |
$$ K+\frac{3}{10} $$ |
$$ K+\frac{4}{10} $$ |
$$ K+\frac{2}{10} $$ |
If the equation of the pair of straight lines passing through the point $(1,1)$ and perpendicular to the pair of lines $3 x^2+11 x y-4 y^2=0$ is $a x^2+2 h x y+b y^2+2 g x+2 f y+12=0$, then $2(a-h+b-g+f-12)=$
Equation of the circle having its centre on the line $2 x+y+3=0$ and having the lines $3 x+4 y-18=0,3 x+4 y+2=0$ as tangents is
If a function $f(x)=\left\{\begin{array}{cl}\frac{\tan (\alpha+1) x+\tan 2 x}{x} & \text { if } x>0 \\ \beta & \text { at } x=0 \text { is } \\ \frac{\sin 3 x-\tan 3 x}{x^3} & \text { if } x<0\end{array}\right.$
continuous at $x=0$, then $|\alpha|+|\beta|=$
$$ \begin{aligned} &\text { If } \int \frac{3}{2 \cos ^3 x \sqrt{2 \sin 2 x}} d x=\frac{3}{2}(\tan x)^B+\frac{3}{10}(\tan x)^A+C \text {, than }\\&A= \end{aligned} $$
$$ \text { } \int\limits_{-3}^3|2-x| d x= $$
$$ \int_{\frac{1}{\sqrt[5]{31}}}^{\frac{1}{\sqrt[5]{242}}} \frac{1}{\sqrt[5]{x^{30}+x^{25}}} d x= $$
$$ \begin{aligned} &\text { The general solution of the differential equation }\\ &(x+y) y d x+(y-x) x d y=0 \text { is } \end{aligned} $$
Physics
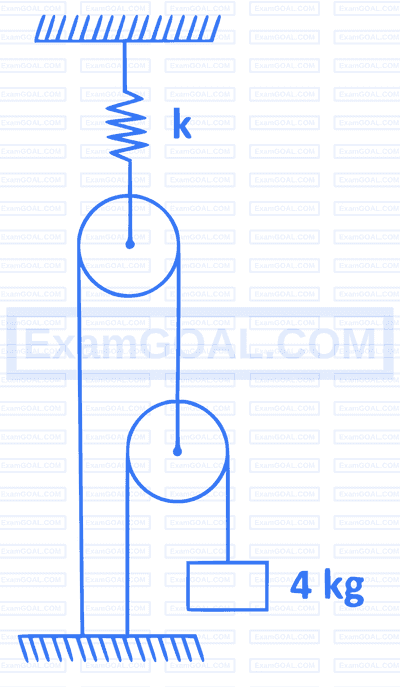
A $4 \mathrm{~kg}$ mass is suspended as shown in figure. All pulleys are frictionless and spring constant $k$ is $8 \times 10^3 \mathrm{Nm}^{-1}$. The extension in spring is $\left(g=10 \mathrm{~ms}^{-2}\right)$
A 3 kg block is connected as shown in the figure. Spring constants of two springs $k_1$ and $k_2$ are $50 \mathrm{Nm}^{-1}$ and $150 \mathrm{Nm}^{-1}$ respectively. The block is released from rest with the springs unstretched. The acceleration of the block in its lowest position is $\left(g=10 \mathrm{~ms}^{-2}\right)$

When the load applied to a wire is increased from 5 kg wt to 8 kg wt . The elongation of the wire increases from 1 mm to 1.8 mm . The work done during the elongation of the wire is (acceleration due to gravity $=10 \mathrm{~ms}^{-2}$ )

