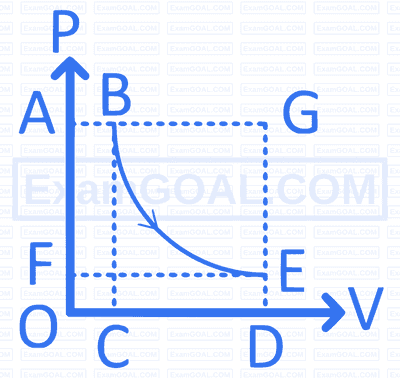
Given below are two statements :
Statement I For isothermal irreversible change of an ideal gas, $q=-w=p_{\text {ext }}\left(V_{\text {final }}-V_{\text {initial }}\right)$
Statement II For adiabatic change, $\Delta U=w_{\text {adiabatic }}$
The correct answer is :




$K_{\mathrm{c}}$ for the following reaction is 99.0
$$ A_2(g) \stackrel{T(K)}{\rightleftharpoons} B_2(g) $$
In a one litre flask, 2 moles of $A_2$ was heated to $T(\mathrm{~K})$ and the above equilibrium is reached. The concentration at equilibrium of $A_2$ and $B_2$ are $C_1\left(A_2\right)$ and $C_2\left(B_2\right)$ respectively. Now, one mole of $A_2$ was added to flask and heated to $T(\mathrm{~K})$ to established the equilibrium again. The concentration of $A_2$ and $B_2$ are $C_3\left(A_2\right)$ and $C_4\left(B_2\right)$ respectively. what is the value of $C_3\left(A_2\right)$ in $\mathrm{mol} \mathrm{L}^{-1}$ ?