Redox Reactions · Chemistry · MHT CET
MCQ (Single Correct Answer)
In the equation, $\mathrm{BiO}_3^{-}+6 \mathrm{H}^{+}+x \mathrm{e}^{-} \rightarrow \mathrm{Bi}^{3+}+3 \mathrm{H}_2 \mathrm{O}$ What is the value of $x$ ?
What is the charge required to convert $2 \mathrm{~mol} \mathrm{KMnO}_4$ to $\mathrm{MnSO}_4$ ?
What is oxidation number of sulphur in $\mathrm{SO}_3$ ?
Which of the following species acts as an weakest reducing agent?
What is the oxidation number of phosphorus in calcium phosphate?
For a reaction, $\mathrm{NH}_4 \mathrm{NO}_2 \longrightarrow \mathrm{~N}_2+2 \mathrm{H}_2 \mathrm{O}$. Which from following phenomena is true regarding nitrogen?
Identify correct statement for reaction stated below.
$$ \mathrm{Ag}_2 \mathrm{O}+\mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Ag}+2 \mathrm{OH}^{-} $$
A compound of Xe and F is found to have atomic ratio $\mathrm{Xe}: \mathrm{F}$ as $0.4: 2.4$, Find the oxidation number of Xe ?
Nitric oxide reacts with $\mathrm{H}_2$ according to reaction. $2 \mathrm{NO}_{(\mathrm{g})}+2 \mathrm{H}_{2(\mathrm{~g})} \rightarrow \mathrm{N}_{2(\mathrm{~g})}+2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})}$.
Identify the correct relationship for consumption of reactant and formation of product.
Which of the following species acts as strongest oxidising agent?
What is the difference in oxidation number of Mn in $\mathrm{KMnO}_4$ and $\mathrm{MnO}_2$ ?
Identify correct statement for the reaction.
$$ \mathrm{I}_2+\mathrm{KClO}_3 \xrightarrow{\Delta} \mathrm{ICl}+\mathrm{KIO}_3 $$
What is the number of electrons transferred considering Mn when $\mathrm{KMnO}_4$ is converted into $\mathrm{Mn}_2 \mathrm{O}_3$ ?
Which is NOT correct regarding stock notation?
If a reaction occurs in following two steps
i. $2 \mathrm{ClO}^{-} \longrightarrow \mathrm{ClO}_2^{-}$
ii. $\mathrm{ClO}_2^{-}+\mathrm{ClO}^{-} \longrightarrow \mathrm{ClO}_3^{-}+\mathrm{Cl}^{-}$
Find the reaction intermediate.
A compound contains atoms of $x, y$ and z elements. Oxidation numbers of $x, y$ and $z$ are +3 , +5 and -2 respectively. Identify the possible formula of this compound.
Which metal in following compounds is not present in fractional oxidation state?
Which of the following is a redox reaction?
What are the respective oxidation states of sulphur atoms numbered 1 to 4 in tetrathionate ion shown below?
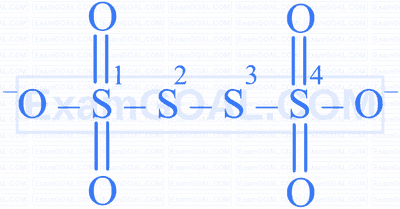
What is the oxidation number of Mn in $\mathrm{MnO}_4^{-}$?
What is the oxidation state of phosphorus in phosphate ion?
What is change in oxidation number of nitrogen when $\mathrm{NO}_3^{-}$is converted to $\mathrm{NH}_4^{+}$ion?
Calculate the oxidation number of Cr in $\mathrm{CrO}_4^{2-}$ ion and $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ respectively.
Identify the correct statement for following reaction.
$$3 \mathrm{Mg}+\mathrm{N}_2 \longrightarrow \mathrm{Mg}_3 \mathrm{~N}_2$$
What is oxidation number of S in $\mathrm{SO}_3^{2-}$ ?
What is the change in oxidation number of S in following reaction?
$$\mathrm{H}_2 \mathrm{~S}+\mathrm{NO}_3^{-} \longrightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{NO}+\mathrm{S}$$
What is the oxidation number of carbon in $\mathrm{K}_2 \mathrm{C}_2 \mathrm{O}_4 ?$
What is the oxidation state of iodine in $\mathrm{I}_2 \mathrm{Cl}_6$ ?
Which of the following changes involves transfer of 5 electrons?
What is the value of $x$ and $y$ in order to balance following redox reaction?
$$\mathrm{xCuO}+\mathrm{yNH}_3 \longrightarrow \mathrm{xCu}+\mathrm{N}_2+\mathrm{xH}_2 \mathrm{O}$$
What is the oxidation state of S in $\mathrm{SO}_4^{2-}$ ?
Which compound from following contains iodine with highest oxidation number?
In which of the following compounds chlorine has highest oxidation state?
Identify the element reduced in following reaction.
$$\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{I}^{-} \longrightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{H}_2 \mathrm{O}+3 \mathrm{I}_2$$
What is the oxidation number of underlined species in $\mathrm{PF}_6^{-}$and $\mathrm{V}_2 \mathrm{O}_7^{-4}$ ions respectively?
Which among the following species is reduced by tin easily?
What is the number of moles of electrons gained by one mole oxidizing agent in following redox reaction?
$$\mathrm{Zn_{(s)}+2HCl_{(aq)}}$$ $$\longrightarrow$$ $$\mathrm{ZnCl_2+H_2}$$
Which from following elements exhibits usual tendency to undergo reduction?
What is the change in oxidation number of selenium in the following redox reaction?
$$\mathrm{SeO}_{3(\text { (a) })}^{2-}+\mathrm{Cl}_{2(\mathrm{~g})}+2 \mathrm{OH}^{-} \longrightarrow \mathrm{SeO}_{4(\text { (a) })}^{2-}+2 \mathrm{Cl}_{(\text {(a) })}^{-}+\mathrm{H}_2 \mathrm{O}$$
What is the change in oxidation number of $$\mathrm{Cr}$$ in the following redox reaction?
$$3 \mathrm{H}_2 \mathrm{O}_{2(\mathrm{aq})}+\mathrm{Cr}_2 \mathrm{O}_{7 \text { (aq) }}^{2-}+8 \mathrm{H}_{(\mathrm{aq})}^{+} \longrightarrow 3 \mathrm{O}_{2(\mathrm{~g})}+2 \mathrm{Cr}^3+7 \mathrm{H}_2 \mathrm{O}$$
What is the oxidation number of sulfur in $$\mathrm{H}_2 \mathrm{SO}_5$$ ?
Identify the elements undergoing reduction and oxidation respectively in the following redox reaction.
$$3 \mathrm{H}_3 \mathrm{AsO}_{3(\mathrm{aq})}+\mathrm{BrO}_{3(\mathrm{aq})}^{-} \rightarrow \mathrm{Br}_{(\mathrm{aq})}^{-}+3 \mathrm{H}_3 \mathrm{AsO}_4$$
What is the value of $$x$$ in order to balance following redox reaction?
$$\mathrm{Mn}_{(\mathrm{aq})}^{2+}+x \mathrm{ClO}_{3(\mathrm{aq})}^{-} \rightarrow \mathrm{MnO}_{2(\mathrm{~s})}+x \mathrm{ClO}_{2(\mathrm{aq})}$$
What is the oxidation state of carbon in $$\mathrm{CaC}_2$$ and $$\mathrm{K}_2 \mathrm{C}_2 \mathrm{O}_4$$ respectively?
Which one of the following conversions does NOT involve either oxidation or reduction?
Which among the following reactions exhibits the reducing property of ozone?
Which of following is NOT a redox reaction?
What is the change in oxidation number of nitrogen in following conversion?
$$\mathrm{NO}_3^{-} \longrightarrow \mathrm{NO}_2$$
Identify reducing agent in following reaction
$$\mathrm{H}_2 \mathrm{O}_{2(\mathrm{ag})}+\mathrm{ClO}_{4(\mathrm{aq})}^{-} \rightarrow \mathrm{ClO}_{2(\mathrm{aq})}^{-}+\mathrm{O}_{2(\mathrm{g})}$$
Identify reductant in following reaction.
$$\mathrm{H}_2 \mathrm{~S}+\mathrm{NO}_2 \rightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{NO}+\mathrm{S}$$
Oxidation state of Cr in potassium dichromate is
What is the value of '$$\mathrm{x}$$' in order to balance the following redox reaction by ion electron method? $$\quad \mathrm{x} \mathrm{H}_2 \mathrm{O}_2+\mathrm{ClO}_4 \rightarrow \mathrm{xO}_2+\mathrm{ClO}_2+2 \mathrm{H}_2 \mathrm{O}$$
Identify reductant in following reaction.
$$\mathrm{{C_2}O_4^{2 - } + MnO_4^ - + {H^ + } \longrightarrow M{n^{2 + }} + C{O_2} + {H_2}O}$$
What is the oxidation number of Mn in MnO$^{2-}_4$ ion?
Which among the following is used as an oxidising agent to bleach wood pulp into white paper?
What is the oxidation number of As in H$$_3$$AsO$$_3$$ ?
Which of the following oxides can act both as an oxidising agent as well as reducing agent?
Oxidation state of nitrogen in nitric oxide is
In which among the following compounds, oxidation number of nitrogen is +5 ?
The oxidation number of sulphur in $\mathrm{S}_8$ molecule is
The oxidation state of sulphur in $\mathrm{H}_2 \mathrm{S}_2 \mathrm{O}_7$ is
The ionic charges of manganate and permanganate ion are respectively
$$\begin{aligned} & \text { In the reaction, } \\ & \mathrm{MnO}_4^{-1}(a q)+\mathrm{Br}^{-1}(a q) \longrightarrow \mathrm{MnO}_2(s)+\mathrm{BrO}_3^{-1}(a q) \end{aligned}$$
,the correct change in oxidation number of the species involved is