Alcohol, Phenols and Ethers · Chemistry · KCET
MCQ (Single Correct Answer)
Phenol can be distinguished from propanol by using the reagent
$$ \text { Match the following with their } \mathrm{pKa} \text { values } $$
| Acid | pKa |
| (I) Phenol | (a) 16 |
| (II) p-Nitrophenol | (b) 0.78 |
| (III) Ethyl alcohol | (c) 10 |
| (IV) Picric acid | (d) 7.1 |

$$ A \text { and } B \text { respectively are } $$
8.8 g of monohydric alcohol added to ethyl magnesium iodide in ether liberates $2240 \mathrm{~cm}^3$ of ethane at STP. This monohydric alcohol when oxidised using pyridinium-chloro-chromate, forms a carbonyl compound that answers silver mirror test (Tollen's test). The monohydric alcohol is
When a tertiary alcohol ' $A^{\prime}\left(\mathrm{C}_4 \mathrm{H}_{10} \mathrm{O}\right)$ reacts with $20 \% \mathrm{H}_3 \mathrm{PO}_4$ at 358 K , it gives a compound ' $B^{\prime}\left(\mathrm{C}_4 \mathrm{H}_8\right)$ as a major product. The IUPAC name of the compound ' $B$ ' is
PCC is
$$\begin{aligned} & \mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_2 \mathrm{OH} \stackrel{\mathrm{PCC}}{\longrightarrow} \\\\ & \mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CHO} \end{aligned}$$
Hybridisation change involved at $$\mathrm{C}$$-$$1$$ in the above reaction.
A better reagent to oxidise primary alcohols into aldehyde is

Y in the above reaction is
An organic compound with molecular formula $$\mathrm{C}_7 \mathrm{H}_8 \mathrm{O}$$ dissolves in $$\mathrm{NaOH}$$ and gives a characteristic colour with $$\mathrm{FeCl}_3$$. On treatment with bromine, it gives a tribromo derivative $$\mathrm{C}_7 \mathrm{H}_5 \mathrm{OBr}_3$$. The compound is
In Kolbe's reaction the reacting substances are
The major product obtained when ethanol is heated with excess of conc. $$\mathrm{H}_2 \mathrm{SO}_4$$ at $$443 \mathrm{~K}$$ is
Among the following, the products formed by the reaction of anisole with $$\mathrm{HI}$$ are
Which is most VISCOUS?
A compound $$'A' \left(\mathrm{C}_7 \mathrm{H}_8 \mathrm{O}\right)$$ is insoluble in $$\mathrm{NaHCO}_3$$ solution but dissolve in $$\mathrm{NaOH}$$ and give a characteristic colour with neutral $$\mathrm{FeCl}_3$$ solution. When treated with bromine water compound '$$A$$' forms the compound $$B$$ with the formula $$\mathrm{C}_7 \mathrm{H}_5 \mathrm{OBr}_3$$. '$$A$$' is
The major product of the following reaction is
$$\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2-\mathrm{OH} \xrightarrow[\text { Excess }]{\mathrm{HBr}} \text { Product }$$
Which of the following on heating gives an ether as major products?
(P) $$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}+\mathrm{CH}_3 \mathrm{ONa}$$
(Q) $$\mathrm{C}_6 \mathrm{H}_5 \mathrm{ONa}+\mathrm{CH}_3 \mathrm{Br}$$
(R) $$\left(\mathrm{CH}_3\right)_3 \mathrm{C}-\mathrm{Cl}+\mathrm{CH}_3 \mathrm{ONa}$$
(S) $$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}=\mathrm{CHCl}+\mathrm{CH}_3 \mathrm{ONa}$$
The steps involved in the conversion of propan -2-ol to propan -1-ol are in the order
Match the following acids with their $$pKa$$ values :
| Acid | $$pKa$$ | ||
|---|---|---|---|
| A. | Phenol | i. | 16 |
| B. | $$p$$-Nitrophenol | ii. | 0.78 |
| C. | Ethanol | iii. | 10 |
| D. | Picric acid | iv. | 7.1 |
Which of the following can be used to test the acidic nature of ethanol?
What is the increasing order of acidic strength among the following?
(i) p-methoxy phenol
(ii) $p$-methyl phenol
(iii) $p$-nitro phenol

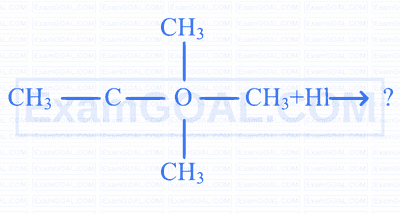
In the following sequence of reactions
$$ \mathrm{CH}_3 \mathrm{Br} \xrightarrow{\mathrm{KCN}} A \xrightarrow{\mathrm{H}_3 \mathrm{O}^{-}} B \xrightarrow{\mathrm{LiAl} / \mathrm{H}_4} $$
The end product $C$ is