Chemistry
1. Identify the element having highest value of first ionization enthalpy. 2. One mole of a perfect gas expands isothermally and reversibly from $10 \mathrm{dm}^3$ to $20 \mathrm{dm}^3$ at 300 K . F 3. Identify anionic complex from following. 4. Identify a ligand having two donor atoms and uses pairs of electrons on both donor atoms. 5. $$ \text { What is IUPAC name of following compound? } $$ 6. Which from the following buffers is used for precipitation of cations of IIIA group in qualitative analysis? 7. Which of the following has the highest basic strength? 8. The molar conductivity of 0.02 M HCl is $408.4 \Omega^{-1} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$ at $25^{\circ} \mathrm{C}$ 9. What is the percentage by mass of oxygen in water, if percentage by mass of hydrogen is $11.1 \%$ ? 10. Identify a pair of compounds that exhibits functional group isomerism from following. 11. What is the order of a reaction having unit of the rate constant $\mathrm{mol} \mathrm{dm}^{-3} \mathrm{~s}^{-1}$ ? 12. Alkyl chlorides on heating with metal fluorides form alkyl fluorides. This reaction is known as
13. Which of the following is NOT an example of diamagnetic substance? 14. Identify the product formed in the following reaction.
$$ \mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_2-\mathrm{CH 15. Identify the major product formed when 2-chlorobutane is boiled with concentrated alcoholic KOH . 16. Which of the following substances is NOT required in the construction of $\mathrm{H}_2-\mathrm{O}_2$ fuel cell? 17. Which alkali metal is used for heat transfer in nuclear power station? 18. Which of the following solvents is safe to use and considered as green solvent? 19. What is rate constant of a first order reaction having half life 138.6 minute? 20. Which of the following compounds has highest boiling point? 21. Calculate the volume of bcc unit cell if the radius of an atom in it is 216.5 pm .
22. Which of the following statements is NOT true about lyophobic colloids? 23. Identify the correct increasing order of boiling point of following compounds. 24. Identify the product ' B ' in the following sequence of reactions.
Ethanenitrile $\xrightarrow{\mathrm{SnCl}_2, \mathrm{ 25. Find increase in temperature for a gas when first its pressure and then volume both are doubled at 400 K . 26. Calculate heat of formation of $\mathrm{SO}_2$ from following equations.
$$ \begin{aligned} & \mathrm{S}+\frac{3}{2} \ma 27. If four different elements $\mathrm{A}, \mathrm{B}, \mathrm{C}$ and D having outer electronic configuration as,
$$ \math 28. Which from the following statements about nylon 2-nylon 6 is NOT correct? 29. Which carbon atoms of glucose in open chain structure numbered from $\mathrm{C}-1$ to $\mathrm{C}-6$ are NOT chiral? 30. The dissociation constant of weak monoacidic base is $1.8 \times 10^{-5}$. Calculate the degree of dissociation in 0.02 31. What is the expected value of $\Delta T_f$ for 0.2 m aqueous $\mathrm{CaCl}_2$ solution if $\Delta \mathrm{T}_{\mathrm{f 32. How many isomers of $\mathrm{C}_4 \mathrm{H}_{11} \mathrm{~N}$ are tertiary amines? 33. Calculate the number of moles of electrons required to convert $1.1 \mathrm{~mol} \mathrm{Cr}_2 \mathrm{O}_7^{2-}$ to $\ 34. Which from following metal ions in their respective oxidation states forms colourless compounds? 35. Which of the following symbols represents heat of reaction at constant pressure? 36. Which from following amino acids contains sulfur in it's side chain? 37. Which from following polymers is obtained by addition polymerisation method? 38. Calculate the pH of buffer solution containing 0.12 M weak acid and 0.48 M of its salt with strong base if $\mathrm{pK}_ 39. Calculate $\Delta \mathrm{T}_{\mathrm{b}}$ of 0.02 m solution if molal elevation constant for solvent is $0.52 \mathrm{~ 40. Identify IUPAC name of the following compound. 41. What is the representation of element ( X ) having mass number 40 containing 22 neutrons. 42. Identify oxidant in following reaction.
$$ 3 \mathrm{H}_3 \mathrm{AsO}_{3(\mathrm{aq})}+\mathrm{BrO}_{3(\mathrm{aq})}^{- 43. Identify false statement about zinc. 44. For the reaction,
$$ 3 \mathrm{I}_{(\mathrm{aq})}^{-}+\mathrm{S}_2 \mathrm{O}_{8(\mathrm{aq})}^{2-} \longrightarrow 2 \m 45. Which of the following is a tertiary allylic alcohol? 46. Calculate the number of atoms in 0.4 gram of metal if it forms fcc structure.
$$ \left[\rho \times \mathrm{a}^3=1.2 \tim 47. Identify a molecule having octahedral geometry as per VSEPR theory. 48. Calculate the mass of solute dissolved in $1 \mathrm{dm}^3$ water has osmotic pressure 3.0 atm at 300 K [Molar mass of s 49. Identify functional group from following that forms pink colour when schiffs reagent is added in it.
50. What is the radius of first orbit of monopositive helium ion?
Physics
1. The centre of mass of a system of particles does NOT depend on 2. A blackbody at $1227^{\circ} \mathrm{C}$ emits radiation with maximum intensity at a wavelength of $5600 $$\mathop {\rm{ 3. A resistor of $400 \Omega$, an inductance of 0.4 H are in series with an a.c. source of e.m.f. $\mathrm{E}=200 \sqrt{2} 4. A plane wavefront of width ' $x$ ' is incident on an air-water interface and the corresponding refracted wavefront has a 5. The intermediate image formed by an objective lens of a compound microscope is 6. A solid sphere and a ring have equal mass and equal radius of gyration. If sphere is rotating about its diameter and rin 7. The third overtone of a closed pipe of length ' $\mathrm{L}_{\mathrm{c}}$ ' has the same frequency as the third overtone 8. To manufacture a solenoid of length ' $l$ ' and inductance ' $L$ ', the length of the thin wire required is (cross - sec 9. A soap bubble of radius $R$ is surrounded by another soap bubble of radius $2 R$ as shown in figure. The excess pressure 10. For a transistor, the current amplification factor ' $\alpha$ ' is 0.8 . The transistor is then connected in common emit 11. Three charges $Q,-2 q$ and $-2 q$ are placed at the vertices of an isosceles right-angled triangle as shown in figure. T 12. A spring has length ' $L$ ' and force constant ' $K$ '. It is cut into two springs of length ' $\mathrm{L}_1$ ' and ' $\ 13. The period of revolution of planet A around the sun is 8 times that of $B$. The distance of $A$ from the sun is how many 14. In Young's double slit experiment, fringe width is 1.4 mm with light of wavelength $6000 $$\mathop {\rm{A}}\limits^{\rm{ 15. Radioactive materials A and B have decay constants ' $9 \lambda$ ' and ' $\lambda$ ' respectively. Initially they have s 16. Two bodies A and B have their moments of inertia ' I ' and ' 2 I ' respectively about their axis of rotation. If their k 17. The flux linked with the coil at any instant ' t ' is given by $\phi=12 t^2-60 t+275$. The magnitude of induced e.m.f. a 18. Two sounding sources send waves at certain temperature in air of wavelength 60 cm and 60.6 cm respectively. The frequenc 19. An ideal gas of mass ' $M$ ' is in the state ' $A$ ' goes to another state B via three different processes. If $Q_1, Q_2 20. The magnitude of magnetic induction at a point on the axis at a large distance ' $r$ ' from the centre of a circular coi 21. In the series LCR circuit shown in figure, the impedance is 22. An ideal gas is heated from $27^{\circ} \mathrm{C}$ to $627^{\circ} \mathrm{C}$ at constant pressure. If initial volume 23. A large insulated sphere of radius ' $r$ ', charged with ' $Q$ ' units of electricity, is placed in contact with a small 24. A thin concavo-convex lens with convex face receiving incident rays has radii of curvatures 12 cm and 24 cm respectively 25. At two points on a horizontal tube of varying cross-section the radii are 1 cm and $\mathbf{0 . 4 ~ c m}$, velocities of 26. A simple harmonic progressive wave is given by equation $y=\operatorname{asin} 2 \pi\left(n t-\frac{x}{\lambda}\right)$. 27. $$ \text { In the following figure, the current } \mathrm{I} \text { is equal to } $$
28. The work function of a photosensitive metallic surface is $h v_0$. If photons of energy (2.5) $h v_0$ fall on this surfa 29. A magnet having a magnetic dipole moment ' M is placed in two magnetic fields ' $\mathrm{B}_1$ ' and ' $\mathrm{B}_2$ ' 30. Two objects A and B are separated horizontally by distance ' a '. Object B moves in a direction perpendicular to distanc 31. Pressure of the gas remaining same, the temperature at which r. m. s. speed of the gas molecules is double its value at 32. In a single slit diffraction experiment, slit of width ' a ' and incident light of wavelength $5600 $$\mathop {\rm{A}}\l 33. If $R_1$ and $R_2$ are the radii of the atomic nuclei of mass numbers 27 and 125 respectively, then the ratio $R_2: R_1$ 34. By increasing the temperature, the electrical conductivity of a conductor and a semiconductor, 35. Water rises up to height ' $x$ ' in a capillary tube immersed vertically in water. When the whole arrangement is taken t 36. Three tuning forks $\mathrm{A}, \mathrm{B}$ and C have respective frequencies $\mathrm{n}_1, \mathrm{n}_2$ and $\mathrm{ 37. In the Davisson Germer experiment, the velocity of electrons emitted from the electron gun can be increased by 38. A circular coil carrying current ' $I$ ' has radius ' $R$ ' and magnetic field at the centre is ' $B$ '. At what distanc 39. A satellite of mass ' $m$ ' is orbiting the earth of radius ' $R$ ' at height ' $h$ ' from the surface of earth. The tot 40. Rods ' $A$ ', ' $B$ ' and ' $C$ ' are made of a paramagnetic, a ferromagnetic and a diamagnetic substance respectively. 41. To get an output $\mathrm{Y}=1$ in the given logic circuit, the correct choice for the input is 42. Which one of the following statements is false for a particle moving in a circle with constant angular speed? 43. An alternating current is given by $\mathrm{I}=100 \sin (50 \pi t)$. How many times will the current become zero in one 44. A particle starts from mean position and performs S.H.M. with period 6 second. At what time its kinetic energy is $50 \% 45. Rate of flow of heat through a cylindrical rod is $\mathrm{H}_1$. The temperature of the ends of the rod are ' $\mathrm{ 46. A coil of area $12 \mathrm{~cm}^2$ has 250 turns. Magnetic field of $0.2 \mathrm{~Wb} / \mathrm{m}^2$ is perpendicular t 47. For which of the following combinations of working temperatures, the efficiency of Carnot's engine is maximum? 48. A galvanometer of resistance ' $G$ ' is converted into an ammeter of resistance $\frac{\mathrm{G}}{40}$, by connecting a 49. The wrong statement out of the following statements is 50. The potentials at points A and B are $\mathrm{V}_{\mathrm{A}}$ and $\mathrm{V}_{\mathrm{B}}$ respectively for the charge
1
MHT CET (PCB) 2024 22th April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Which of the following is a tertiary allylic alcohol?
A
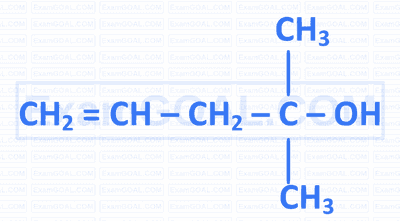
B
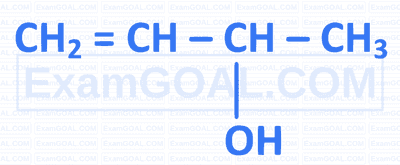
C

D

2
MHT CET (PCB) 2024 22th April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Calculate the number of atoms in 0.4 gram of metal if it forms fcc structure.
$$ \left[\rho \times \mathrm{a}^3=1.2 \times 10^{-22} \mathrm{~g}\right] $$
A
$\quad 2.516 \times 10^{22}$
B
$1.333 \times 10^{22}$
C
$3.721 \times 10^{22}$
D
$4.905 \times 10^{22}$
3
MHT CET (PCB) 2024 22th April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Identify a molecule having octahedral geometry as per VSEPR theory.
A
$\quad \mathrm{PCl}_5$
B
$\mathrm{CH}_4$
C
$\quad \mathrm{BeCl}_2$
D
$\mathrm{SF}_6$
4
MHT CET (PCB) 2024 22th April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Calculate the mass of solute dissolved in $1 \mathrm{dm}^3$ water has osmotic pressure 3.0 atm at 300 K [Molar mass of solute $=41 \mathrm{~g} \mathrm{~mol}^{-1}$, $\mathrm{R}=0.082 \mathrm{dm}^3 \mathrm{~atm} \mathrm{~K} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ ]
A
4.00 g
B
5.00 g
C
6.20 g
D
7.25 g
Paper analysis
Total Questions
Chemistry
50
Physics
50
More papers of MHT CET (Biology)
MHT CET (Biology)
Papers