Biology
In eukaryotic organisms, replication of DNA occurs in _________
Capillarity theory was proposed by __________
Given below are two statements.
Statement I - Oxaloacetate is useful as a precursor for synthesis of aspartic acid.
Statement II - $\alpha$-ketoglutarate is useful for synthesis of glutamic acid.
In light of above statements, select the correct answer from the option given below.
Given below are two statements.
Statement I - Defecation is an involuntary process that takes place through anal opening guarded by sphincter muscles.
Statement II - Distension, of rectum stimulates pressure sensitive receptors that initiate a neural reflex for egestion.
In light of above statements, select the correct answer from the option given below.
The term 'Niche' denotes the _________ role played by an organism in its environment.
When the phage DNA enters the host bacterium, the host cell protects itself from the viral DNA attacks with the help of _________ enzyme.
Given below are two statements.
Statement I - Nervous tissue is without lymphatic vessels.
Statement II - Nervous tissue is endodermal in origin.
In light of above statements, choose the most appropriate correct answer from the option given below.
With reference to adolescence, what is mhGAP?
Damage to biodiversity takes place due to natural reasons which includes the following EXCEPT
| Column - I | Column - II | ||
|---|---|---|---|
| i. | Thalassemia | a. | X monosomy |
| ii. | Turner's syndrome | b. | Extra X chromosomes in male |
| iii | Klinefelter's syndrome | c. | $$ 21^{\text {st }} \text { Trisomy } $$ |
| iv. | Down's syndrome | d. | Mendelian disorder |
Chemical digestion of which one of the following starts first in human alimentary canal?
DDT resistant mosquitoes and different beak sizes of African seed cracker finches show
_________ type of selection respectively.
The number of ATP molecules generated in aerobic respiration after oxidation of two molecules of pyruvic acid is.
During oogenesis the meiotic division is arrested at________ before fertilization.
Accessory cells of stomata are reservoirs of_________.
Mature erythrocytes are enucleated in following animals EXCEPT
i. Rat
ii. Monkey
iii. Llama
iv. Camel
v. Elephant
Choose the correct answer from options given below.Agarose gel electrophoresis is used to separate DNA fragments. The DNA fragments separate due to __________ .
How many of the following are the symbiotic nitrogen fixing micro-organisms?
Rhizobium, Anabaena, Frankia, Azotobacter, Nostoc, Clostridium, Beijerinckia, Klebsiella
CSF is secreted by
Match the scientist in column I with their contribution in column II and choose the correct option.
| Column - I | Column - II | ||
|---|---|---|---|
| i. | Alexander Van Humboldt | a. | Estimated 7 million species round the globe |
| ii. | Rober ay | b. | Rivet popper hypothesis |
| iii | David TIllman | c. | Species Area Relationship |
| iv. | Paul Ehrlich | d. | Productivity stability hypothesis |
Given below are two statements:
Statement I - During night, guard cells become flaccid.
Statement II - During day time, guard cells become turgid due to endosmosis.
In light of above statements, choose the most appropriate answer from the option given below.
Which one of the following is an EXCEPTION to the generalization made by Mendel on the basis of his experiments on garden pea plant?
People who consume high protein diet can develop __________ stones.
In an angiospermic embryo, upper swollen suspensor cell towards the micropyle, functions as_________.
In large proteins, peptide chains are much looped, twisted and folded back on themselves. The folded structure is due to the formation of________bonds
Arrange the following in sequence during the transmission of nerve impulse at chemical synapse.
i. Neurotransmitter binds with receptors of post synaptic cell.
ii. Calcium channels open and the calcium diffuses inward from ECF.
iii. Impulse travels along the axon of presynaptic neuron.
iv. Release of neurotransmitter by exocytosis.
v. Synaptic vesicles fuse with cell membrane of axon terminal of presynaptic neuron.
The highest level of ecological hierarchy in ecological organization is
Primary productivity in an ecosystem refers to the rate of generation of _________ .
Cells of malignant tumor spread from one organ to other via blood or lymph and cause new tumor. This property is called ___________ .
Haemoglobin has maximum affinity for
Given below are two statements:
Statement I - In prokaryotes, there is only replicon however in eukaryotes, these are several replicons in tandem.
Statement II - Two separated strands in a replicon of DNA are prevented from re-joining by helicase enzyme.
In light of above statements, choose the correct answer from the option given below.
Which of the following characteristics are seen in a stable community?
i. It is strong enough to withstand environmental disturbance and recovers quickly.
ii. It is resistant to invasive species.
iii. It exhibits constant change in biomass production over a period of time.
iv. It has more species diversity.
Given below are two statements:
Statement I - Back cross is the crossing of $F_1$ hybrid with one of the two parents from which they were derived.
Statement II - Test cross involves the crossing of $F_1$ - hybrid with its homozygous recessive parent.
In light of above statements, choose the correct answer from the option given below.
Complete the analogy with respect to structure of sperm
Nebenkern : ' X ' : : Acrosome is formed from : ' Y '
Given below are two statements:
Statement I - The muscularis of intestine can be differentiated into longitudinal and oblique muscles only.
Statement II - The muscularis of stomach is made up of outer- longitudinal, middle oblique and inner circular muscles.
In light of above statements, choose the most appropriate answer from the option given below.
An organ with sphincters at its origin is
The size of mRNA is generally related to $\qquad$
Given below are two statements:
Statement I - Reactions involved in Krebs cycle are anabolic and catabolic.
Statement II - During oxidation of acetyl Co-A, stepwise oxidation of acetyl part of acetyl Co-A occurs.
In light of above statements, choose the most appropriate answer from the option given below.
Match the valves of human heart in column I with the respective opening they guard in column II and select the correct option.
| Column - I | Column - II | ||
|---|---|---|---|
| i. | Eustachian valve | a. | Opening of pulmonary aorta |
| ii. | Thebesian valve | b. | Left atrioventricular aperture |
| iii | Mitral valve | c. | opening of inferior vena cava |
| iv. | Semilunar valve | d. | opening of coronary sinus |
The _______ gene from Bacillus thuringiensis produces a protein that forms crystalline inclusions in bacterial spores.
Lateral sulcus separates ________ .
Which of the following is INCORRECT about Primary and Secondary ecological successions?
Following are viral diseases in poultry, EXCEPT ________
All the 64 codons in the dictionary of genetic code were deciphered by __________
Median vertical depression of external genitalia in females, enclosing the urethral and vaginal opening is _________
Bird pollinated flowers are usually __________
Complete the following analogy.
Parietal wall of Bowman's capsule : squamous epithelium : : PCT : ___________ epithelium.
Complete the following reaction by replacing (Y) with proper word.
$$ \text { Emulsified fats } \xrightarrow{\text { Lipases }} \text { Fatty acids }+(\mathrm{Y}) $$
Complete the following analogy with respect to classification of mammals.
$$ \text { Marsupials : (i) :: monotremes : (ii) } $$
While studying flowering behaviour in __________ and ___________ plant, Garner and Allard discovered photoperiodism.
Which of the following bacteria do not solubilize the insoluble rock phosphate?
Atherosclerosis can be treated with recombinant protein called __________
Match elements given in Column I with their deficiency symptoms shown by plants given in Column II
| Column - I | Column - II | ||
|---|---|---|---|
| i. | Eustachian valve | a. | Brown heart disease |
| ii. | Boron | b. | Malformed leaves |
| iii | Zinc | c. | Poor growth of the plant |
| iv. | Copper | c. | Die-back of shoots |
Given below are two statements.
Statement I - Biome constitute, a large regional terrestrial unit delimited by a specific climate zone having major vegetation zone and associated fauna.
Statement II - Biome is the fourth level of ecological hierarchy.
In light of above statements, select the correct answer from the option given below.
Two different amino acids are never encoded by the same codon, this character of genetic code is called ____________.
Given below are two statements.
Statement I - Root hair is composed of two layers.
Statement II - Outer layer of root hair cell wall is composed of cellulose and inner layer is made up of pectin.
In light of above statements, choose the most appropriate answer from the option given below.
The fully developed foetus gives signals for uterine contractions by secreting __________ hormones.
Just before fertilization, the angiosperm's embryo sac contains_________ and ___________nuclei.
Just before fertilization, the angiosperm's embryo sac contains ________ and ________ nuclei.
Given below are two statements.
Statement I - An ecosystem is a self regulatory and self sustaining structural and functional unit of nature.
Statement II - Entire biosphere can be considered as one global ecosystem.
In light of above statements, choose the most appropriate answer from the options given below.
The number of reduced coenzymes $\mathrm{NADH}+\mathrm{H}^{+}$ formed during complete oxidation of one molecule of glucose.
Monohybrid crosses are useful in demonstrating which of the following laws of inheritance suggested by Mendel?
Enzymes are needed only in small quantities to catalyse reactions because
The number of amino groups in the amino acids present in amides is __________
Which of the following are the initial and final steps during the process of ecological succession?
Match column I with column II and select the correct option
| Column - I | Column - II | ||
|---|---|---|---|
| i. | Carrot and spinach | a. | High protein content |
| ii. | Bitter gourd | b. | More Vit A and minerals |
| iii | Maize | c. | More Vit C |
| iv. | Wheat Atlas-66 |
c. | Twice the amount of lysine and tryptophan |
Hypersecretion of glucocorticoid leads to___________
The restriction enzymes used as molecular scissors are type of ____________
Given below are two statements.
Statement I - Calotropis growing in abandoned fields is never consumed by cattle and goats.
Statement II - Calotropis produces highly poisonous cardiac glycosides.
In light of above statements, select the most appropriate answer from the option given below.
Phenotypically tall plants can be obtained from genetically dwarf maize plants by application of_________
Islets of Langerhans are _________ .
Homo sapiens are most closely related to________
Complete the analogy with respect to reproductive system.
Male : Penis : : Female : __________ .
In a male gametophyte, ploidy of tube cell and a male gamete is __________
In which of the following, bilirubin is excreted in urine?
The only 5C intermediate formed during reactions of TCA cycle is __________
Secondary metabolites like glucosinolates are produced by cabbage to
Tubular fluid tends to become acidic in the_________ of nephron.
In an antibody, disulphide bonds are present___________
i. between two heavy chains
ii. between two light chains
iii. between the constant region of light chain and the constant region of a heavy chain
iv. between antigen binding sites of both the heavy and light chains
v. between the variable region of light chain and that of heavy chain
Select the correct answer from the options given below
The water which percolates deep in the soil is ___________
Select INCORRECT statements with respects transport of $\mathrm{CO}_2$ by RBCs and plasma.
i. $23 \%$ of $\mathrm{CO}_2$ released from tissue cells diffuse into plasma first and then into RBCs.
ii. Within RBCs carbonic acid immediately dissociates into $\mathrm{H}^{+}$and $\mathrm{HCO}_3^{-}$.
iii. In RBCs $\mathrm{CO}_2$ combines with $\mathrm{H}_2 \mathrm{O}$ to form carbonic acid.
iv. $\mathrm{H}^{+}$ions move out of RBCs and combine with $\mathrm{Na}^{+}$to form $\mathrm{NaHCO}_3$.
v. $7 \%$ of $\mathrm{CO}_2$ is transported in dissolved form as carbonic acid in plasma.
In the light of above statements choose the correct answer from option given below.
Dispersal of pollens is an example of
Given below are two statements:
Statement I - Toddy is made by fermenting fleshy pedicels of cashews nuts.
Statement II - Fenny is made by fermenting the sugar sap extracted from palm plants and coconut palm.
In light of above statements, select the correct answer from the option given below.
Which one of the following pair of hormones is NOT antagonistic?
In the processing of hnRNA in eukaryotic cell, the primary transcripts are processed in the following sequence.
Hormone causing vigorous contraction of myometrium to initiate parturition is ___________ .
Which plant hormone is called antitranspirant?
Blood osmolarity decreases with the secretion of
i. ADH
ii. aldosterone
iii. angiotensin II
iv. ANP
Blood plasma transports oxygen in dissolved state, percentage of which is ________ .
Which of the following statements are correct?
i. $X$ chromosome has large amount of euchromatin.
ii. heterochromatin is genetically inert.
iii. Both X and Y chromosomes are homologous.
iv. Crossing over does not take place in sex chromosomes in female.
Choose the correct option.
Identify label ' X ' from the given diagram and name the cells, which regulate concentration of calcium and phosphorous in the blood and select the correct option given below.
Which one of the following is the most primitive ancestor of Homo sapiens?
Match column I with column II and select the correct option.
| Column I | Column II | ||
| i. | Frontal lobe | a. | Gustato receptors |
| ii. | Parietal lobe | b. | Olfactory receptors |
| iii. | Temporal lobe | c. | Sense of vision |
| iv. | Occipital lobe | d. | Broca's area |
Which one of the following diseases is caused by a fungus?
Respiratory centres that control the rate and depth of breathing are located in __________
In sea grass, pollens are long, ribbon like and without exine exhibiting __________ pollination.
Chemistry
The entropy of vaporisation of benzene is $85 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$. When 117 g of benzene vaporises at its boiling point, what is entropy change of surrounding if process is at equilibrium?
Which from the following equations represents the relation between solubility ( $\mathrm{mol} \mathrm{L}^{-1}$ ) and solubility product for the salt $\mathrm{B}_2 \mathrm{~A}$ ?
Calculate the Henry's law constant at $25^{\circ} \mathrm{C}$ if solubility of gas in liquid is $2.1 \times 10^{-2} \mathrm{~mol} \mathrm{dm}^{-3}$ at 0.18 bar.
What is the name of isopropyl alcohol according to carbinol system?
What is the frequency of red light having wave length 750 nm ?
Identify the product ' $C$ ' formed in the following series of reactions.

Which from following groups exhibits +I effect?
Which from following statements is NOT true about physisorption?
Which from following is a semisynthetic polymer?
Calculate the molar mass of an element having density $19.2 \mathrm{~g} \mathrm{~cm}^{-3}$ if it forms fcc structure $\left[\mathrm{a}^3 \times \mathrm{N}_{\mathrm{A}}=40 \mathrm{~cm}^3 \mathrm{~mol}^{-1}\right]$
Which among the following is the strongest acid?
What is the ratio of mass of nitrogen that combines with 16 parts by weight of oxygen in $\mathrm{N}_2 \mathrm{O}, \mathrm{NO}, \mathrm{NO}_2$ ?
What is the quantity of electricity required to liberate $112 \mathrm{~cm}^3$ of hydrogen gas at STP from acidified water?
Which of the following is selected as cathode for a galvanic cell set up with nickel anode?
$$ \text { Which among the following is haloarene? } $$
Which from following chemical activities does NOT exhibit good atom economy according to the principles of green chemistry?
Calculate activation energy for a reaction if it's rate doubles when temperature is raised from $20^{\circ} \mathrm{C}$ to $35^{\circ} \mathrm{C}\left(\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right)$
What is the formal charge present on oxygen atom (numbered 1) in Lewis structure of $\mathrm{CO}_2$ ?

Which among the following is a monomer of natural rubber?
Identify the product obtained when alkyl alkanoate is hydrolysed with dilute HCl .
The molar conductivity of $0.02 \mathrm{~mol} \mathrm{dm} \mathrm{dm}^{-3}$ solution of sodium hydroxide is $230.5 \Omega^{-1} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$. What is it's conductivity?
Calculate the volume occupied by particle in simple cubic unit cell if volume of unit cell is $5.5 \times 10^{-22} \mathrm{~cm}^3$.
Which of the following is primary benzylic alcohol?
If half life of a first order reaction is 10 minutes, find the time required to decrease concentration of reactant from 0.08 M to 0.02 M .
Calculate the pOH of buffer solution formed from 0.3 M weak base and 0.45 M of its salt with strong acid $\left[\mathrm{pK}_{\mathrm{b}}=4.7447\right]$
Which from following complexes is having ambidentate ligand in it?
How many moles of iodomethane are consumed in the following conversion?
$$ \mathrm{CH}_3 \mathrm{NH}_2 \xrightarrow[\Delta]{\mathrm{CH}_3 \mathrm{l}}\left(\mathrm{CH}_3\right)_4 \mathrm{~N}^{+} \mathrm{I}^{-} $$
Identify the use of calcium carbonate from following.
Identify false statement about transition elements.
Which from following terms is explained by first law of thermodynamics?
Calculate degree of dissociation of a weak monobasic acid in 0.01 M solution if dissociation constant is $1.6 \times 10^{-5}$.
Which from following is an example of solution of solid as solute and liquid as solvent?
Identify the coordination number of aluminium in potassium trioxalatoaluminate(III).
Which of the following isomeric amines has the highest boiling point?
If $\mathrm{A}, \mathrm{B}, \mathrm{C}, \mathrm{D}$ are four different elements with outer electronic configuration as $\mathrm{A}=4 \mathrm{~s}^2 4 \mathrm{p}^4, \mathrm{~B}=4 \mathrm{~s}^2 4 \mathrm{p}^5, \mathrm{C}=5 \mathrm{~s}^2 5 \mathrm{p}^4, \mathrm{D}=5 \mathrm{~s}^2 5 \mathrm{p}^5$.
Find the element having highest ionization enthalpy $\left(\Delta_1 \mathrm{H}_1\right)$
What is the change in internal energy for $2 \mathrm{CO}_{(\mathrm{g})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{CO}_{2(\mathrm{~g})}$ at $25^{\circ} \mathrm{C}$ ?
( $\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}, \Delta \mathrm{H}=-560 \mathrm{~kJ}$ )
Calculate the final pressure required to reduce the volume of a gas to one third, if initial pressure is $1.6 \times 10^5 \mathrm{Nm}^{-2}$.
If osmotic pressure of 0.2 M aqueous glucose solution is 5 atm at 300 K . Calculate the concentration of glucose solution having osmotic pressure 2.5 atm at same temperature.
What is the IUPAC name of following compound?
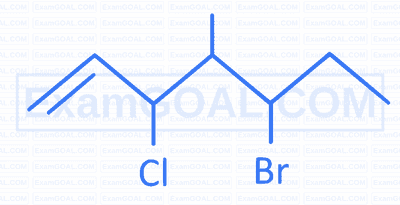
Which amino acid from following contains highest number of N atoms in it?
Identify the reagent ' R ' necessary to bring the following conversion.

$$ \text { Identify ' } A \text { ' in the following reaction. } $$

Physics
Water is flowing through a horizontal pipe in a streamline flow. At the narrowest part of the pipe
Two different coils have self - inductance $\mathrm{L}_1=9 \mathrm{mH}$ and $\mathrm{L}_2=3 \mathrm{mH}$. The current in first coil is increased at a constant rate. The current in the second coil is also increased at the same constant rate. At certain instant of time, the power given to the two coils is same. At that time, there was current and induced voltage in the two coils. At the same instant, the ratio of the energy stored in the first coil to that in second coil is
Four masses of $1 \mathrm{~kg}, 2 \mathrm{~kg}, 3 \mathrm{~kg}$ and 4 kg are kept at co-ordinates $(0,0) \mathrm{m},(0,1) \mathrm{m}$ and $(1,0) \mathrm{m}$ respectively. Using the co-ordinates of centre of mass its position vector is
In between the plates of parallel plate capacitor of plate separation 'd' a dielectric plate of thickness ' $t$ ' is inserted. The capacitance becomes one-third of the original capacity without dielectric. The dielectric constant of the plate is
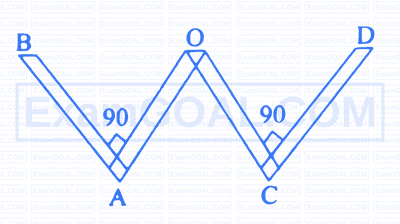
A thin rod of length ' 4 L ' and mass ' 4 m ' is bent at the points as shown in the figure. The moment of inertia of the rod about an axis passing through point ' $O$ ' and perpendicular to plane of the paper is
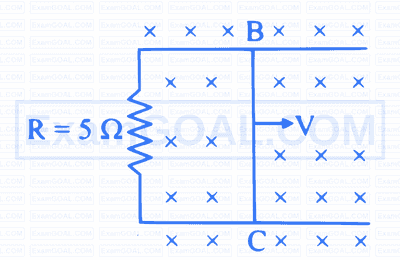
The resistance of a coil for d.c. is $5 \Omega$. In a.c., the resistance will
When a ray of light is refracted from one medium to another, then the wavelength changes from $6000 \mathop {\rm{A}}\limits^ \circ$ to $4000 \mathop {\rm{A}}\limits^ \circ$. The critical angle for the interface will be
A pipe closed at one end vibrating in fifth overtone is in unison with open pipe vibrating in its fifth overtone. The ratio of $l_{\mathrm{c}}: l_{\mathrm{o}}$ is $\left[l_{\mathrm{c}}=\right.$ vibrating length of closed pipe, $l_0=$ vibrating length of open pipe]
A hollow charged metal sphere has a radius ' $r$ '. If the potential difference between its surface and a point at a distance ' 3 r ' from the centre is ' $v$ ', then the electric field intensity at a distance ' $3 r$ ' is
For a given medium, the speed of light and the polarising angle are ' $v$ ' and ' $i_p$ ' respectively, then ( $\mathrm{c}=$ speed of light in vaccum )
A particle of mass ' m ' is executing S.H.M. about the origin on $x$-axis with frequency $\sqrt{\frac{\mathrm{ka}}{\pi \mathrm{m}}}$, where ' $k$ ' is a constant and ' $a$ ' is the amplitude of S.H.M. If ' x ' is a displacement of a particle, at time ' $t$ ', potential energy of the particle will be
The angular momentum of electron in hydrogen atom in first orbit is ' $L$ '. The change in angular momentum if electron is in second orbit of hydrogen atom is
Four point masses, each of mass ' $m$ ' are arranged in $X-Y$ plane as shown in the figure. The moment of inertia of this system about $X$-axis is

In biprism experiment the maximum intensity is ' $\mathrm{I}_0$ '. If the path difference between the two interfering waves is ' $\lambda / 4$ ' then intensity at the point on the screen is
$$ \left[\sin 45^{\circ}=\cos 45^{\circ}=\frac{1}{\sqrt{2}}\right] $$
Work done to get ' $n$ ' spherical drops of equal size from a single spherical drop of water, is proportional to
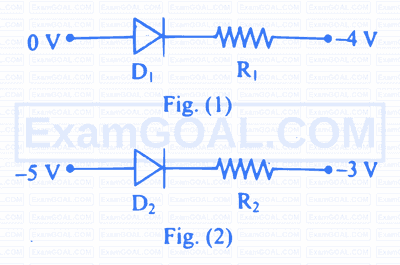
Diode and resistance are connected as shown in figure. Out of the following statements which one is TRUE?
Two uniform strings ' $A$ ' and ' $B$ ' made of steel are made to vibrate under same tension. If the first overtone of ' $A$ ' is equal to second overtone of ' B ' and radius of ' A ' is twice that of ' B '. Then the ratio of length of string ' $A$ ' to that of ' $B$ ' is
Which one of the following is the correct equation for the electric circuit shown in the figure?

A pendulum is performing simple harmonic motion. The acceleration of the bob is $20 \mathrm{~cm} \mathrm{~s}^{-2}$ at a distance of 5 cm from mean position. The time period of oscillation is
A spherical body of radius ' $r$ ' radiates power ' $P$ ' at $T$ kelvin. If the radius $s$ halved and the temperature doubled the power radiated in the same time ' t ' will be
A stone is thrown upward with a speed 'u' from the top of a tower reaches the ground with velocity ' $3 u$ '. The height of the tower is ( $g=$ acceleration due to gravity)
A metal surface is illuminated by light of two different wavelengths 207 nm and 414 nm . The maximum speeds of photoelectrons corresponding to these wavelengths are $\mathrm{u}_1$ and $\dot{u}_2$ respectively with $u_1: u_2=2: 1$. The work function of the metal is $(\mathrm{hc}=1242 \mathrm{eV} \mathrm{nm})$
In thermodynamic process, which of the following statements is not true?
A charged particle is subjected to acceleration in a cyclotron which consists of two dees ' $\mathrm{D}_1$ ' and ' $D_2$ '. The charged particle undergoes increase in its speed.
In case of well of death which is a vertical cylindrical wall of radius ' $r$ ' inside which vehicle is driven in horizontal circles. If ' $m$ ' is mass of vehicle, ' $V$ ' is the velocity and ' $\mu_s$ ' is the coefficient of static friction between the wheels of vehicle and walls then correct relation is [ $\mathrm{g}=$ acceleration due to gravity]
In an oscillating LC circuit, the maximum charge on the capacitor is ' $Q$ '. When the energy is stored equally between the electric and magnetic fields, the instantaneous charge on the capacitor ' $q$ ' is
Two particles execute S.H.M. of same amplitude and frequency along the same straight line path. They pass each other when going in opposite directions, each time their displacement is half the amplitude. The phase difference between them is $\left(\sin 30^{\circ}=0 \cdot 5\right)$
The pitch of whistle of an engine appears to drop by $30 \%$ of the original value when it passes a stationary observer. If speed of sound in air is $350 \mathrm{~m} / \mathrm{s}$, then the speed of engine in $\mathrm{m} / \mathrm{s}$ is
A monoatomic ideal gas, initially at temperature ' $\mathrm{T}_1$ '' is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature ' $\mathrm{T}_2$ ' by releasing the piston suddenly. $L_1$ and $L_2$ are the lengths of the gas columns before and after the expansion respectively. Then $\frac{T_1}{T_2}$ is
In the diagram, the total electric flux through the closed surface ' S ' is
[Given $\mathrm{q}=$ charge
$\varepsilon_0=$ permittivity of free space]
If an electron in hydrogen atom jumps from $3^{\text {rd }}$ orbit to $2^{\text {nd }}$ orbit it emits a photon of wavelength ' $\lambda$ '. When it emits a photon from $4^{\text {th }}$ orbit to $3^{\text {rd }}$ orbit then the corresponding wavelength of emitted photon will be
A solenoid having 400 turns/metre has a core of a material with relative permeability 300 . If a current of 0.5 A is passed through it, then the magnetisation of the core material is nearly
The volume of a block of metal at $30^{\circ} \mathrm{C}$ changes by $0.12 \%$ when its temperature is increased to $70^{\circ} \mathrm{C}$. The coefficient of linear expansion of the metal is
A galvanometer of resistance ' $G$ ' can be converted into a voltmeter of range ( $0-\mathrm{V}$ ) volt by connecting a resistance ' $R$ ' in series with it. The resistance ' $R$ required to change its range from $\left(0-\frac{\mathrm{V}}{4}\right)$ volt will be
A light metal disc of radius ' $r$ ' floats on water surface and bends the surface downwards along the perimeter making an angle ' $\theta$ ' with the vertical edge of the disc. If the weight of water displaced by the disc is ' W ', the weight of the metal disc is [ $\mathrm{T}=$ surface tension of water]
The magnetic field at the centre of a current carrying circular coil of area ' $A$ ' is ' $B$ '. The magnetic moment of the coil is ( $\mu_0=$. permeability of free space)
A parallel combination of two capacitors of capacities ' C ' and ' $\frac{\mathrm{C}}{3}$, respectively is connected across a battery of 12 volt. When both capacitors are fully charged, the charge and energy stored in them is $Q_1, Q_2$ and $E_1, E_2$ respectively. Then the ratio of $\left(E_1-E_2\right)$ to $\left(Q_1-Q_2\right)$ is
A transparent sphere of refractive index ' $\mu$ ' and radius of curvature ' $R$ ' is kept in air. A point object is placed at a distance ' d ' from the surface of the sphere so that the real image is formed at the same distance ' $d$ ' from exactly opposite side of the sphere. The distance ' $d$ ' is
The instantaneous value of current in an a.c. circuit is $I=2 \sin \left[100 \pi t+\frac{\pi}{3}\right]$ A. The current will be maximum for the first time at $\left(\sin 90^{\circ}=1\right)$
A fixed mass of gas at constant pressure occupies a volume ' $V$ '. The gas undergoes a rise in temperature so that the r.m.s. velocity of the molecule is doubled. The new volume will be
The Boolean expression for $\mathrm{X}-\mathrm{OR}$ gate $C=(A \oplus B)$ is equivalent to
The equation of simple harmonic progressive wave is given by $y=A \sin (100 \pi t-4 x)$. The distance between two particles having a phase difference of $\left(\frac{\pi}{4}\right)^c$ is
A photon and an electron have an equal energy ' E '. The ratio of wavelength ' $\lambda_{\mathrm{p}}$ ' of photon to that of electron ' $\lambda_{\mathrm{e}}$ ' is proportional to
The height at which the acceleration due to gravity becomes $\frac{g}{4}$ in terms of $R$ is $[R=$ the radius of the earth $]$
In a biprism experiment, fifth dark fringe is obtained at a point. A thin transparent film of refractive index ' $\mu$ ' is placed in one of the interfering paths. Now $7^{\text {th }}$ bright fringe is obtained at the same point. If ' $\lambda$ ' is the wavelength of light used, the thickness of film is equal to
Figure shows a rectangular frame situated in a constant magnetic field. A wire BC of length 1 m is moved out with velocity $4 \mathrm{~m} / \mathrm{s}$. Magnetic field strength is 0.15 T . Force acting on the wire $B C$ is