Chemistry
1. The exact order of boiling points of the compounds n-pentane, isopentane, butanone and 1-butanol is 2. The maximum number of atoms that can be in one plane in the molecule p-nitrobenzonitrile are 3. Cyclo [18] carbon is an allotrope of carbon with molecular formula C18. It is a ring of 18 carbon atoms, connected by si 4. p-nitro-N, N-dimethylaniline cannot be represented by the resonating structures. 5. The relationship between the pair of compounds shown above are respectively 6. The exact order of acidity of the compounds p-nitrophenol, acetic acid, acetylene and ethanol is 7. The dipeptides which may be obtained from the amino acids glycine, and alanine are 8. The compounds A and B above are respectively. 9. For a spontaneous reaction at all temperatures which of the following is correct? 10. A given amount of Fe2+ is oxidised by x mol of $$MnO_4^ - $$ in acidic medium. The number of moles of $$C{r_2}O_7^{2 - } 11. An element crystallises in a body centered cubic lattice. The edge length of the unit cell is 200 pm and the density of 12. Molecular velocities of two gases at the same temperature (T) are u1 and u2. Their masses are m1 and m2 respectively. Wh 13. When 20 g of naphthoic acid (C11H8O2) is dissolved in 50 g of benzene, a freezing point depression of 2K is observed. Th 14. The equilibrium constant for the reaction N2(g) + O2(g) $$\rightleftharpoons$$ 2NO(g) is 4 $$\times$$ 10$$-$$4 at 2000 K 15. Under the same reaction conditions, initial concentration of 1.386 mol dm$$-$$3 of a substance becomes half in 40 s and 16. Which of the following solutions will have highest conductivity? 17. Indicate the products (X) and (Y) in the following reactionsNa2S + nS(n = 1 $$-$$ 8) $$\to$$ (X)Na2SO3 + S $$\to$$ (Y) 18. 2.5 mL 0.4 M weak monoacidic base (Kb = 1 $$\times$$ 10$$-$$12 at 25$$^\circ$$C) is titrated with 2/15 M HCl in water at 19. Solubility products (Ksp) of the salts of types MX, MX2 and M3X at temperature T are 4.0 $$\times$$ 10$$-$$8, 3.2 $$\tim 20. The reduction potential of hydrogen half-cell will be negative if 21. A saturated solution of BaSO4 at 25$$^\circ$$C is 4 $$\times$$ 10$$-$$5 M. The solubility of BaSO4 in 0.1 M Na2SO4 at th 22. A solution is made by a concentrated solution of Co(NO3)2 with a concentrated solution of NaNO2 is 50% acetic acid. A so 23. Extraction of a metal (M) from its sulphide ore (M2S) involves the following chemical reactions$$2{M_2}S + 3{O_2}\buildr 24. The white precipitate (Y), obtained on passing colorless and odourless gas (X) through an ammoniacal solution of NaCl, l 25. Which structure has delocalised $$\pi$$-electrons? 26. The H3O+ ions has the following shape 27. For the reaction $$_7^{14}N(\alpha ,p)\,{}^{17}O$$, 1.16 MeV (Mass equivalent = 0.00124 amu) of energy is absorbed. Mass 28. A solution of NaNO3, when treated with a mixture of Zn dust and 'A' yields ammonia. 'A' can be 29. Indicate the number of unpaired electrons in K3[Fe(CN)6] and K4[Fe(CN)6]. 30. Which of the following compounds have magnetic moment identical with [Cr(H2O)6]3+ ? 31. Among the following chlorides the compounds which will be hydrolysed most easily and most slowly in aqueous NaOH solutio 32. The products X and Y which are formed in the following sequence of reactions are respectively. 33. The atomic masses of helium and neon are 4.0 and 20.0 amu respectively. The value of the de-Broglie wavelength of helium 34. The mole fraction of a solute in a binary solution is 0.1 at 298 K, molarity of this solution is same as its molality. D 35. 5.75 mg of sodium vapour is converted to sodium ion. If the ionisation energy of sodium is 490 kJ mol$$-$$1 and atomic w 36. The product(s) in the following sequence of reactions will be 37. The compounds X and Y are respectively 38. Aqueous solution of HNO3, KOH, CH3COOH and CH3COONa of identical concentration are provided. The pair(s) of solutions wh 39. Reaction of silver nitrate solution with phosphorus acid produces 40. N2H4 and H2O2 show similarity in
Mathematics
1. If $$I = \mathop {\lim }\limits_{x \to 0} sin\left( {{{{e^x} - x - 1 - {{{x^2}} \over 2}} \over {{x^2}}}} \right)$$, the 2. Let f : R $$\to$$ R be such that f(0) = 0 and $$\left| {f'(x)} \right| \le 5$$ for all x. Then f(1) is in 3. If $$\int {{{\sin 2x} \over {{{(a + b\cos x)}^2}}}dx} = \alpha \left[ {{{\log }_e}\left| {a + b\cos x} \right| + {a \ov 4. Let $$g(x) = \int\limits_x^{2x} {{{f(t)} \over t}dt} $$ where x > 0 and f be continuous function and f(2x) = f(x), then 5. $$\int\limits_1^3 {{{\left| {x - 1} \right|} \over {\left| {x - 2} \right| + \left| {x - 3} \right|}}dx} $$ is equal to 6. The value of the integral $$\int\limits_{ - {1 \over 2}}^{{1 \over 2}} {{{\left\{ {{{\left( {{{x + 1} \over {x - 1}}} \r 7. If $$\int\limits_{{{\log }_e}2}^x {{{({e^x} - 1)}^{ - 1}}dx = {{\log }_e}{3 \over 2}} $$, then the value of x is 8. The normal to a curve at P(x, y) meets the X-axis at G. If the distance of G from the origin is twice the abscissa of P 9. The differential equation of all the ellipses centred at the origin and have axes as the co-ordinate axes is where $$y^{ 10. If $$x{{dy} \over {dx}} + y = {{xf(xy)} \over {f'(xy)'}}$$, then | f(xy) | is equal to (where k is an arbitrary positive 11. The straight the through the origin which divides the area formed by the curves y = 2x $$-$$ x2, y = 0 and x = 1 into tw 12. The value of $$\int\limits_0^5 {\max \{ {x^2},6x - 8\} \,dx} $$ is 13. A bulb is placed at the centre of a circular track of radius 10 m. A vertical wall is erected touching the track at a po 14. Two particles A and B move from rest along a straight line with constant accelerations f and f' respectively. If A takes 15. let $$\alpha$$, $$\beta$$, $$\gamma$$ be three non-zero vectors which are pairwise non-collinear. if $$\alpha$$ + 3$$\be 16. Let f : R $$\to$$ R be given by f(x) = | x2 $$-$$ 1 |, x$$\in$$R. Then, 17. Let a, b, c be real numbers, each greater than 1, such that $${2 \over 3}{\log _b}a + {3 \over 5}{\log _c}b + {5 \over 2 18. Consider the real valued function h : {0, 1, 2, ...... 100} $$\to$$ R such that h(0) = 5, h(100) = 20 and satisfying h(p 19. If |z| = 1 and z $$\ne$$ $$\pm$$ 1, then all the points representing $${z \over {1 - {z^2}}}$$ lie on 20. Let C denote the set of all complex numbers. Define A = {(z, w) | z, w$$\in$$C and |z| = |w|}, B = {z, w} | z, w$$\in$$C 21. Let $$\alpha$$, $$\beta$$ be the roots of the equation x2 $$-$$ 6x $$-$$ 2 = 0 with $$\alpha$$ > $$\beta$$. If an = $$\a 22. For x$$\in$$R, x $$\ne$$ $$-$$1, if $${(1 + x)^{2016}} + x{(1 + x)^{2015}} + {x^2}{(1 + x)^{2014}} + ..... + {x^{2016}} 23. Five letter words, having distinct letters, are to be constructed using the letters of the word 'EQUATION' so that each 24. What is the number of ways in which an examiner can assign 10 marks to 4 questions, giving not less than 2 marks to any 25. The digit in the unit's place of the number 1! + 2! + 3! + .... + 99! is 26. If M is a 3 $$\times$$ 3 matrix such that (0, 1, 2) M = (1 0 0), (3, 4 5) M = (0, 1, 0), then (6 7 8) M is equal to 27. Let $$A = \left( {\matrix{
1 & 0 & 0 \cr
0 & {\cos t} & {\sin t} \cr
0 & { - \sin t} & {\cos t} \cr
} } 28. Let A and B two non singular skew symmetric matrices such that AB = BA, then A2B2(ATB)$$-$$1(AB$$-$$1)T is equal to 29. If an (> 0) be the nth term of a G.P. then$$\left| {\matrix{
{\log {a_n}} & {\log {a_{n + 1}}} & {\log {a_{n + 2}}} 30. Let A, B, C be three non-void subsets of set S. Let (A $$\cap$$ C) $$\cup$$ (B $$\cap$$ C') = $$\phi$$ where C' denote t 31. Let T and U be the set of all orthogonal matrices of order 3 over R and the set of all non-singular matrices of order 3 32. Four persons A, B, C and D throw and unbiased die, turn by turn, in succession till one gets an even number and win the 33. The mean and variance of a binomial distribution are 4 and 2 respectively. Then the probability of exactly two successes 34. Let $${S_n} = {\cot ^{ - 1}}2 + {\cot ^{ - 1}}8 + {\cot ^{ - 1}}18 + {\cot ^{ - 1}}32 + ....$$ to nth term. Then $$\math 35. If a > 0, b > 0 then the maximum area of the parallelogram whose three vertices are O(0, 0), A(a cos$$\theta$$, b sin$$\ 36. Let A be the fixed point (0, 4) and B be a moving point on X-axis. Let M be the midpoint of AB and let the perpendicular 37. A moving line intersects the lines x + y = 0 and x $$-$$ y = 0 at the points A, B respectively such that the area of the 38. The locus of the vertices of the family of parabolas $$6y = 2{a^3}{x^2} + 3{a^2}x - 12a$$ is 39. A ray of light along $$x + \sqrt 3 y = \sqrt 3 $$ gets reflected upon reaching X-axis, the equation of the reflected ray 40. Two tangents to the circle x2 + y2 = 4 at the points A and B meet at M($$-$$4, 0). The area of the quadrilateral MAOB, w 41. From a point (d, 0) three normal are drawn to the parabola y2 = x, then 42. If from a point P(a, b, c), perpendicular PA and PB are drawn to YZ and ZX-planes respectively, then the equation of the 43. The co-ordinate of a point on the auxiliary circle of the ellipse x2 + 2y2 = 4 corresponding to the point on the ellipse 44. The locus of the centre of a variable circle which always touches two given circles externally is 45. A line with positive direction cosines passes through the point P(2, $$-$$1, 2) and makes equal angle with co-ordinate a 46. For $$y = {\sin ^{ - 1}}\left\{ {{{5x + 12\sqrt {1 - {x^2}} } \over {13}}} \right\};\left| x \right| \le 1$$, if $$a(1 - 47. f(x) is real valued function such that 2f(x) + 3f($$-$$x) = 15 $$-$$ 4x for all x$$\in$$R. Then f(2) = 48. Consider the functions f1(x) = x, f2(x) = 2 + loge x, x > 0. The graphs of the functions intersect 49. The equation 6x + 8x = 10x has 50. Let f : D $$\to$$ R where D = [$$-$$0, 1] $$\cup$$ [2, 4] be defined by $$f(x) = \left\{ {\matrix{
{x,} & {if} & {x \ 51. Let f(x) be continuous periodic function with period T. Let $$I = \int\limits_a^{a + T} {f(x)\,dx} $$. Then 52. If $$b = \int\limits_0^1 {{{{e^t}} \over {t + 1}}dt} $$, then $$\int\limits_{a - 1}^a {{{{e^{ - t}}} \over {t - a - 1}}} 53. The differential of $$f(x) = {\log _e}(1 + {e^{10x}}) - {\tan ^{ - 1}}({e^{5x}})$$ at x = 0 and for dx = 0.2 is 54. Given that f : S $$\to$$ R is said to have a fixed point at c of S if f(c) = c. Let f : [1, $$\infty$$) $$\to$$ R be def 55. The $$\mathop {\lim }\limits_{x \to \infty } {\left( {{{3x - 1} \over {3x + 1}}} \right)^{4x}}$$ equals 56. The area bounded by the parabolas $$y = 4{x^2},y = {{{x^2}} \over 9}$$ and the straight line y = 2 is 57. If a($$\alpha$$ $$\times$$ $$\beta$$) + b($$\beta$$ $$\times$$ $$\gamma$$) + c($$\gamma$$ + $$\alpha$$) = 0, where a, b, 58. If the tangent at the point P with co-ordinates (h, k) on the curve y2 = 2x3 is perpendicular to the straight line 4x = 59. The coefficient of a3b4c5 in the expansion of (bc + ca + ab)6 is 60. Three unequal positive numbers a, b, c are such that a, b, c are in G.P. while $$\log \left( {{{5c} \over {2a}}} \right) 61. The determinant $$\left| {\matrix{
{{a^2} + 10} & {ab} & {ac} \cr
{ab} & {{b^2} + 10} & {bc} \cr
{ac} & {bc 62. Let R be the real line. Let the relations S and T or R be defined by $$S = \{ (x,y):y = x + 1,0 63. The plane lx + my = 0 is rotated about its line of intersection with the plane z = 0 through an angle $$\alpha$$. The eq 64. The points of intersection of two ellipses $${x^2} + 2{y^2} - 6x - 12y + 20 = 0$$ and $$2{x^2} + {y^2} - 10x - 6y + 15 = 65. Let $$I = \int_{\pi /4}^{\pi /3} {{{\sin x} \over x}dx} $$. Then 66. If $$\left| {z + i} \right| - \left| {z - 1} \right| = \left| z \right| - 2 = 0$$ for a complex number z, then z is equa 67. $$\left| {\matrix{
x & {3x + 2} & {2x - 1} \cr
{2x - 1} & {4x} & {3x + 1} \cr
{7x - 2} & {17x + 6} & {12x - 68. The remainder when $${7^{{7^{{7^{{{..}^7}}}}}}}$$ (22 time 7) is divided by 48 is 69. Whichever of the following is/are correct? 70. A plane meets the co-ordinate axes t the points A, B, C respectively such a way that the centroid of $$\Delta$$ABC is (1 71. Let P be a variable point on a circle C and Q be a fixed point outside C. If R is the midpoint of the line segment PQ, t 72. $$\mathop {\lim }\limits_{n \to \infty } \left\{ {{{\sqrt n } \over {\sqrt {{n^3}} }} + {{\sqrt n } \over {\sqrt {{{(n + 73. Let $$f(x) = \left\{ {\matrix{
{0,} & {if} & { - 1 \le x \le 0} \cr
{1,} & {if} & {x = 0} \cr
{2,} & {if} & 74. The greatest and least value of $$f(x) = {\tan ^{ - 1}} - {1 \over 2}\,ln \,x\,on\,\left[ {{1 \over {\sqrt 3 }},\sqrt 3 75. Let f and g be periodic functions with the periods T1 and T2 respectively. Then f + g is
Physics
1. A spherical convex surface of power 5 D separates object and image space of refractive indices 1.0 and $$4\over3$$ , res 2. In Young's double slit experiment, light of wavelength $$\lambda$$ passes through the double slit and forms interference 3. A 12.5 eV electron beam is used to bombard gaseous hydrogen at ground state. The energy level upto which the hydrogen at 4. Let r, v, E be the radius of orbit, speed of electron and total energy of electron respectively in H-atom. Which of the 5. What is the value of current through the diode in the circuit given? 6. For the given logic circuit, the output Y for inputs (A = 0, B = 1) and (A = 0, B = 0) respectively are 7. From dimensional analysis, the Rydberg constant can be expressed in terms of electric charge (e), mass (m) and Planck co 8. Three blocks are pushed with a force F across a frictionless table as shown in figure above. Let N1 be the contact force 9. A block of mass m slides with speed v on a frictionless table towards another stationary block of mass m. A massless spr 10. The acceleration versus distance graph for a particle moving with initial velocity 5 m/s is shown in the figure. The vel 11. A simple pendulum, consisting of a small ball of mass m attached to a massless string hanging vertically from the ceilin 12. In case of projectile motion, which one of the following figures represent variation of horizontal component of velocity 13. A uniform thin rod of length L, mass m is lying on a smooth horizontal table. A horizontal impulse P is suddenly applied 14. Centre of mass (CM) of three particles of masses 1 kg, 2 kg and 3 kg lies at the point (1, 2, 3) and CM of another syste 15. A body of density 1.2 $$\times$$ 103 kg/m3 is dropped from rest from a height 1 m into a liquid to density 2.4 $$\times$ 16. Two solid spheres S1 and S2 of same uniform density fall from rest under gravity in a viscous medium and after sometime, 17. In the given figure, 1 represents isobaric, 2 represents isothermal and 3 represents adiabatic processes of an ideal gas 18. If pressure of real gas O2, in a container is given by $$p = {{RT} \over {2V - b}} - {a \over {4{b^2}}}$$, then the mass 19. 300 g of water at 25$$^\circ$$C is added to 100 g of ice at 0$$^\circ$$C. The final temperature of the mixture is 20. The variation of electric field along the Z-axis due to a uniformly charged circular ring of radius a in XY-plane as sho 21. A metal sphere of radius R carrying charge q is surrounded by a thick concentric metal shell of inner and outer radii a 22. Three infinite plane sheets carrying uniform charge densities $$-$$ $$\sigma$$, 2$$\sigma$$, 4$$\sigma$$ are placed para 23. Two point charges +q1 and +q2 are placed a finite distance d apart. It is desired to put a third charge q3 in between th 24. Consider two infinitely long wires parallel to Z-axis carrying same current I in the positive z-direction. One wire pass 25. A thin charged rod is bent into the shape of a small circle of radius R, the charge per unit length of the rod being $$\ 26. For two types of magnetic materials A and B, variation of $$1\over\chi$$ ($$\chi$$ : susceptibility) versus temperature 27. The rms value of potential difference V shown in the figure is 28. The carbon resistor with colour code is shown in the figure. There is no fourth band in the resistor. The value of the r 29. Consider a pure inductive AC circuit as shown in the figure. If the average power consumed is P, then 30. The cross-section of a reflecting surface is represented by the equation x2 + y2 = R2 as shown in the figure. A ray trav 31. For a plane electromagnetic wave, the electric field is given by$$ \overrightarrow{E} = 90\sin (0.5 \times {10^3}x + 1.5 32. Two metal wires of identical dimensions are connected in series. If $$\sigma$$1 and $$\sigma$$2 are the electrical condu 33. A uniform rod of length L pivoted at one end P is freely rotated in a horizontal plane with an angular velocity $$\omega 34. An ideal gas of molar mass M is contained in a very tall vertical cylindrical column in the uniform gravitational field. 35. Under isothermal conditions, two soap bubbles of radii a and b coalesce to form a single bubble of radius c. If the exte 36. A small bar magnet of dipole moment M is moving with speed v along x-direction towards a small closed circular conductin 37. Electric field component of an EM radiation varies with time as E = a (cos$$\omega$$0t + sin$$\omega$$t cos$$\omega$$0t) 38. Consider the p - V diagram for 1 mole of an ideal monatomic gas shown in the figure. Which of the following statements i 39. The potential energy of a particle of mass 0.02 kg moving along X-axis is given by V = Ax (x $$-$$ 4) J, where x is in m 40. A particle of mass m and charge q moving with velocity v enters region-b from region-a along the normal to the boundary
1
WB JEE 2021
MCQ (Single Correct Answer)
+2
-0.5

The mole fraction of a solute in a binary solution is 0.1 at 298 K, molarity of this solution is same as its molality. Density of this solution at 298 K is 2.0 g cm$$-$$3. The ratio of molecular weights of the solute and the solvent (Msolute / Msolvent) is
A
9
B
$${1 \over {9}}$$
C
4.5
D
$${1 \over {4.5}}$$
2
WB JEE 2021
MCQ (Single Correct Answer)
+2
-0.5

5.75 mg of sodium vapour is converted to sodium ion. If the ionisation energy of sodium is 490 kJ mol$$-$$1 and atomic weight is 23 units, the amount of energy needed for this conversion will be
A
1.96 kJ
B
1960 kJ
C
122.5 kJ
D
0.1225 kJ
3
WB JEE 2021
MCQ (More than One Correct Answer)
+2
-0

The product(s) in the following sequence of reactions will be
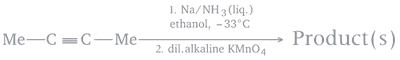
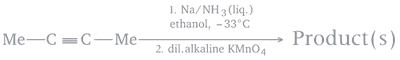
A

B

C

D

4
WB JEE 2021
MCQ (More than One Correct Answer)
+2
-0

The compounds X and Y are respectively
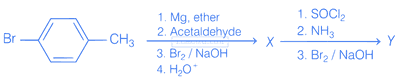

A

B

C

D

Paper analysis
Total Questions
Chemistry
40
Mathematics
75
Physics
40
More papers of WB JEE
WB JEE
Papers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016