Biology
 Which of the following is the most likely pattern of inheritance of the disorder?
Which of the following is the most likely pattern of inheritance of the disorder?
Which of the following proteins plays a direct role in muscle contraction?
$$ \text { Match the list of conditions with the list of affected physiological processes. } $$
| Column I | Column II | ||
|---|---|---|---|
| P | Allergy | i | Excess secretion of growth hormone |
| Q | Uremia | ii | Exaggerated immune response to environmental substances |
| R | Myasthenia gravis | iii | Autoimmune disorder affecting the neuromuscular junction |
| S | Acromegaly | iv | Malfunctioning of kidneys which can lead to urea accumulation in the blood |
Which of the following combinations is correct?
Which of the following graphs represents the correct relationship between light intensity (Xaxis) and the rate of photosynthesis ( $Y$-axis)?
$$ \text { Match the enzymes in Column I with the cellular compartments in Column II. } $$
| Column I | Column II | ||
| P | Succinate dehydrogenase | i | Cytoplasm |
| Q | Pyruvate dehydrogenase | ii | Inner mitochondrial membrane |
| R | Lactate dehydrogenase | iii | Mitochondrial matrix |
| S | ATP synthase | iv | Thylakoid membrane |
| v | Inner chloroplast membrane |
Two species of a flowering plant, $P(2 n=20$ chromosomes) and $Q$ ( $2 n=30$ chromosomes) are reciprocally crossed with each other as male or female as shown below to produce F1 seeds. Which of the following seed tissues from both the F1 seeds ( $R$ and $S$ ) will have the same chromosome numbers?

Which of the following plasmid vectors can be used for cloning of a gene, with restriction enzymes BamHI and EcoRI, and ampicillin-containing nutrient agar for selection? [Ori - origin of replication; $\mathrm{Amp}^R$ - gene for ampicillin resistance]
Polymerase chain reaction (PCR) is used to amplify a gene of interest (GOI). If, after 30 cycles of PCR, 1 billion copies of GOI are produced, approximately how many copies of GOI were present at the end of the $20^{\text {th }}$ cycle?
Which of the following statements is correct about the oxygen $\left(\mathrm{O}_2\right)$ dissociation curves A and C relative to curve B ?

Chemistry
The following complex ions absorb in the ultraviolet-visible region of light. Which one of these shows violet colour?
$$ \left[\mathrm{CoCl}\left(\mathrm{NH}_3\right)_5\right]^{2+},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)\left(\mathrm{NH}_3\right)_5\right]^{3+},\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}, \text { and }\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-} $$
$$ \text { What is the relationship between the structures depicted below? } $$


$$ \text { What are the products } \mathrm{N} \text { and } \mathrm{Q} \text { in the following reaction sequences? } $$

$$ \text { What are } X \text { and } Z \text { in the following sequence of reactions? } $$

$$ \text { What are the correct structural descriptions for } M \text { and } N \text { ? } $$

Consider the following data for KCl solution at a particular temperature. What is the value of the limiting molar conductivity?
$$ \begin{array}{|c|c|} \hline \text { Concentration }\left(\mathrm{mol} \mathrm{~L}^{-1}\right) & \text { Molar Conductivity }\left(\mathrm{S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\right) \\ \hline 1 \times 10^{-4} & 149.1 \\ \hline 9 \times 10^{-4} & 147.1 \\ \hline \end{array} $$
Mathematics
Consider the following lines in the $X Y$-plane:
$$ L_1: 5 x-2 y=1 $$
$L_2$ : The line passing through $(0,1)$ and $(100,101)$,
$L_3$ : The line passing through $(1,11)$ and parallel to the vector $-\hat{\imath}+2 \hat{\jmath}$.
Let $A=\left(L_1 \cap L_2\right) \cup\left(L_2 \cap L_3\right) \cup\left(L_3 \cap L_1\right)$. What is the total number of elements of $A$ ?
Consider the lines $L_1$ and $L_2$ given below:
$$ \begin{gathered} L 1: x=2+\lambda, y=3+2 \lambda, z=4+3 \lambda \\ L 2: x=4+\lambda, y=4, z=4+\lambda \end{gathered} $$
If $(2,3,4)$ is the point of $L_1$ that is closest to $L_2$, then which point of $L_2$ is closest to $L_1$ ?
Consider the following subset of the $X Y$-plane.
$$ S=\left\{\left(|z-\mathrm{i} z|,|z|^2\right): \quad z \text { is a complex number }\right\} $$
Which one of the following statements is correct?
In the given figure, the angles $\angle B A Q=\angle C P Q=\angle C B Q=\frac{\pi}{2}$; and the lengths $Q A=3$ units, $A B=$ 4 units, and $B C=1$ unit. What is the length of $P Q$ ?

Physics
Consider a simple pendulum undergoing simple harmonic motion with a time period $T$, and a fixed amplitude $\theta_0$ of angular oscillation. Its angular momentum about the point of suspension exhibits an oscillatory behaviour with an amplitude $A$. Which of the following relations between $A$ and $T$ is correct?

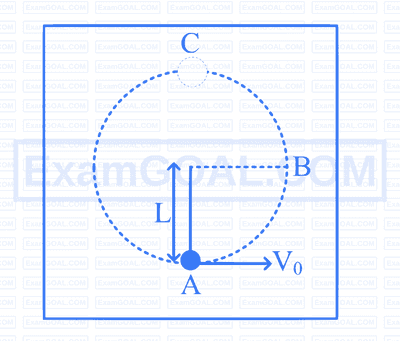
A solid bob of a material having density twice that of water is suspended with a massless and inextensible string of length $L$. The whole set-up is placed inside a water-filled tank. The bob is imparted a horizontal velocity $V_0$ at the lowest point A , while the other end of the string is fixed, such that the bob completes a semi-circular trajectory in the vertical plane. The string becomes slack only when the bob reaches the topmost point C. Assume that the effects of viscosity and water currents are negligible. The acceleration due to gravity is $g$. What is the expression for $V_0$ ?
Consider two point charges $+q$ and $+2 q$ fixed on the $x-y$ plane at $(-\ell / 2,0)$ and $(+\ell / 2,0)$ respectively. Another point charge $-q$ having mass $m$ is released from rest at $(0,(\sqrt{3} / 2) \ell)$ on the $x y$ plane, as shown in the figure The permittivity of free space is $\epsilon_0$. What is the acceleration of the charge $-q$ at the time of release?

Consider the circuit diagram as shown in the figure. The source has a voltage $V=V_0 \sin \omega t$. Both the resistors $A$ and $B$ have the same resistance. The capacitor and the inductor have capacitance $C$ and inductance $L$, respectively. For some frequency $\omega$, and certain initial charge in the capacitor, the current through the resistor $A$ is in phase with the source. What is the value of $\omega$ ?


Consider the shown circuit. The capacitors $C_1$ and $C_2$ have capacitances $2 \mu \mathrm{~F}$ and $8 \mu \mathrm{~F}$, respectively. The switch can connect point $X$ to either $Y$ or $Z$. Initially $X Y$ is connected until the capacitor is fully charged by the battery. Then the switch connects $X$ and $Z$, and the final charges on Cl and C 2 are $Q_1$ and $Q_2$, respectively. What is the value of the ratio $\frac{Q_2}{Q_1+Q_2}$ ?


Two monochromatic sources emit light at wavelengths $\lambda$ and $\lambda / 2$. The stopping potentials for a photosensitive material using these two sources are found to be 1 V and 3 V , respectively. What is the work function of the material?