Biology
1. In eukaryotic organisms, replication of DNA occurs in _________ 2. Capillarity theory was proposed by __________ 3. Given below are two statements.
Statement I - Oxaloacetate is useful as a precursor for synthesis of aspartic acid.
Stat 4. Given below are two statements.
Statement I - Defecation is an involuntary process that takes place through anal opening 5. Angina pectoris is severe pain and heaviness in chest that is mainly caused due to
6. The term 'Niche' denotes the _________ role played by an organism in its environment. 7. When the phage DNA enters the host bacterium, the host cell protects itself from the viral DNA attacks with the help of 8. Given below are two statements.
Statement I - Nervous tissue is without lymphatic vessels.
Statement II - Nervous tissue 9. With reference to adolescence, what is mhGAP? 10. Damage to biodiversity takes place due to natural reasons which includes the following EXCEPT 11. Match the disorder in column I with their nature in column II.
.tg {border-collapse:collapse;border-spacing:0;}
.tg td 12. Chemical digestion of which one of the following starts first in human alimentary canal? 13. DDT resistant mosquitoes and different beak sizes of African seed cracker finches show
_________ type of selection respe 14. The number of ATP molecules generated in aerobic respiration after oxidation of two molecules of pyruvic acid is. 15. During oogenesis the meiotic division is arrested at________ before fertilization. 16. Accessory cells of stomata are reservoirs of_________.
17. Mature erythrocytes are enucleated in following animals EXCEPT
i. Rat
ii. Monkey
iii. Llama
iv. Camel
v. Elephant Choose 18. Agarose gel electrophoresis is used to separate DNA fragments. The DNA fragments separate due to __________ . 19. How many of the following are the symbiotic nitrogen fixing micro-organisms?
Rhizobium, Anabaena, Frankia, Azotobacter, 20. CSF is secreted by 21. Match the scientist in column I with their contribution in column II and choose the correct option.
.tg {border-collap 22. Given below are two statements:
Statement I - During night, guard cells become flaccid.
Statement II - During day time, 23. Which one of the following is an EXCEPTION to the generalization made by Mendel on the basis of his experiments on garde 24. People who consume high protein diet can develop __________ stones. 25. In an angiospermic embryo, upper swollen suspensor cell towards the micropyle, functions as_________. 26. In large proteins, peptide chains are much looped, twisted and folded back on themselves. The folded structure is due to 27. Arrange the following in sequence during the transmission of nerve impulse at chemical synapse.
i. Neurotransmitter bind 28. The highest level of ecological hierarchy in ecological organization is 29. Primary productivity in an ecosystem refers to the rate of generation of _________ . 30. Cells of malignant tumor spread from one organ to other via blood or lymph and cause new tumor. This property is called 31. Haemoglobin has maximum affinity for 32. Given below are two statements:
Statement I - In prokaryotes, there is only replicon however in eukaryotes, these are se 33.
Which of the following characteristics are seen in a stable community?
i. It is strong enough to withstand environment 34. Given below are two statements:
Statement I - Back cross is the crossing of $F_1$ hybrid with one of the two parents fro 35. Complete the analogy with respect to structure of sperm
Nebenkern : ' X ' : : Acrosome is formed from : ' Y ' 36. Given below are two statements:
Statement I - The muscularis of intestine can be differentiated into longitudinal and ob 37. An organ with sphincters at its origin is 38. The size of mRNA is generally related to $\qquad$ 39. Given below are two statements:
Statement I - Reactions involved in Krebs cycle are anabolic and catabolic.
Statement II 40. Match the valves of human heart in column I with the respective opening they guard in column II and select the correct o 41. The _______ gene from Bacillus thuringiensis produces a protein that forms crystalline inclusions in bacterial spores. 42. Lateral sulcus separates ________ . 43. Which of the following is INCORRECT about Primary and Secondary ecological successions? 44. Following are viral diseases in poultry, EXCEPT ________ 45. All the 64 codons in the dictionary of genetic code were deciphered by __________ 46. Median vertical depression of external genitalia in females, enclosing the urethral and vaginal opening is _________ 47. Bird pollinated flowers are usually __________ 48. Complete the following analogy.
Parietal wall of Bowman's capsule : squamous epithelium : : PCT : ___________ epithelium 49. Complete the following reaction by replacing (Y) with proper word.
$$ \text { Emulsified fats } \xrightarrow{\text { Lip 50. Complete the following analogy with respect to classification of mammals.
$$ \text { Marsupials : (i) :: monotremes : (i 51. While studying flowering behaviour in __________ and ___________ plant, Garner and Allard discovered photoperiodism. 52. Which of the following bacteria do not solubilize the insoluble rock phosphate? 53. Atherosclerosis can be treated with recombinant protein called __________ 54. Match elements given in Column I with their deficiency symptoms shown by plants given in Column II
.tg {border-collapse 55. Given below are two statements.
Statement I - Biome constitute, a large regional terrestrial unit delimited by a specifi 56. Identify the INCORRECT statement.
57. Two different amino acids are never encoded by the same codon, this character of genetic code is called ____________. 58. Given below are two statements.
Statement I - Root hair is composed of two layers.
Statement II - Outer layer of root ha 59. The fully developed foetus gives signals for uterine contractions by secreting __________ hormones. 60. Just before fertilization, the angiosperm's embryo sac contains_________ and ___________nuclei. 61. Just before fertilization, the angiosperm's embryo sac contains ________ and ________ nuclei. 62. Given below are two statements.
Statement I - An ecosystem is a self regulatory and self sustaining structural and funct 63. The number of reduced coenzymes $\mathrm{NADH}+\mathrm{H}^{+}$ formed during complete oxidation of one molecule of gluco 64. Monohybrid crosses are useful in demonstrating which of the following laws of inheritance suggested by Mendel? 65. Enzymes are needed only in small quantities to catalyse reactions because 66. The number of amino groups in the amino acids present in amides is __________ 67. Which of the following are the initial and final steps during the process of ecological succession? 68. Match column I with column II and select the correct option
.tg {border-collapse:collapse;border-spacing:0;}
.tg td{ 69. Hypersecretion of glucocorticoid leads to___________
70. The restriction enzymes used as molecular scissors are type of ____________ 71. Given below are two statements.
Statement I - Calotropis growing in abandoned fields is never consumed by cattle and goa 72. Phenotypically tall plants can be obtained from genetically dwarf maize plants by application of_________
73. Islets of Langerhans are _________ . 74. Homo sapiens are most closely related to________
75. Complete the analogy with respect to reproductive system.
Male : Penis : : Female : __________ . 76. In a male gametophyte, ploidy of tube cell and a male gamete is __________ 77. In which of the following, bilirubin is excreted in urine? 78. The only 5C intermediate formed during reactions of TCA cycle is __________ 79. Secondary metabolites like glucosinolates are produced by cabbage to 80. Tubular fluid tends to become acidic in the_________ of nephron.
81. All are events of pollen-pistil interaction EXCEPT
82. Which one of the following cells secrete heparin, histamine and serotonin?
83. In an antibody, disulphide bonds are present___________
i. between two heavy chains
ii. between two light chains
iii. be 84. The water which percolates deep in the soil is ___________
85. Select INCORRECT statements with respects transport of $\mathrm{CO}_2$ by RBCs and plasma.
i. $23 \%$ of $\mathrm{CO}_2$ 86. Dispersal of pollens is an example of 87. Given below are two statements:
Statement I - Toddy is made by fermenting fleshy pedicels of cashews nuts.
Statement II 88. Which one of the following pair of hormones is NOT antagonistic? 89. In the processing of hnRNA in eukaryotic cell, the primary transcripts are processed in the following sequence. 90. Hormone causing vigorous contraction of myometrium to initiate parturition is ___________ . 91. Which plant hormone is called antitranspirant? 92. Blood osmolarity decreases with the secretion of
i. ADH
ii. aldosterone
iii. angiotensin II
iv. ANP 93. Blood plasma transports oxygen in dissolved state, percentage of which is ________ . 94.
Which of the following statements are correct?
i. $X$ chromosome has large amount of euchromatin.
ii. heterochromatin 95. Identify label ' X ' from the given diagram and name the cells, which regulate concentration of calcium and phosphorous 96. Which one of the following is the most primitive ancestor of Homo sapiens? 97. Match column I with column II and select the correct option.
Column I
Column II
i.
Frontal lobe
a.
Gu 98. Which one of the following diseases is caused by a fungus? 99. Respiratory centres that control the rate and depth of breathing are located in __________ 100. In sea grass, pollens are long, ribbon like and without exine exhibiting __________ pollination.
Chemistry
1. Which element from following does NOT exhibit magnetic moment in +1 state?
2. The entropy of vaporisation of benzene is $85 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$. When 117 g of benzene va 3. Which from the following equations represents the relation between solubility ( $\mathrm{mol} \mathrm{L}^{-1}$ ) and sol 4. What is the number of chiral carbon atoms and number of formyl groups respectively present in ribose?
5. Calculate the Henry's law constant at $25^{\circ} \mathrm{C}$ if solubility of gas in liquid is $2.1 \times 10^{-2} \mat 6. What is the name of isopropyl alcohol according to carbinol system? 7. What is the frequency of red light having wave length 750 nm ? 8. Identify the product ' $C$ ' formed in the following series of reactions.
9. Which from following groups exhibits +I effect? 10. Which of the following equation exhibits integrated rate law equation for first order reaction?
11. Which from following statements is NOT true about physisorption? 12. Which from following is a semisynthetic polymer? 13. Calculate the molar mass of an element having density $19.2 \mathrm{~g} \mathrm{~cm}^{-3}$ if it forms fcc structure $\l 14. Which among the following is the strongest acid? 15. What is the ratio of mass of nitrogen that combines with 16 parts by weight of oxygen in $\mathrm{N}_2 \mathrm{O}, \math 16. What is the quantity of electricity required to liberate $112 \mathrm{~cm}^3$ of hydrogen gas at STP from acidified wate 17. Which of the following is selected as cathode for a galvanic cell set up with nickel anode? 18. $$ \text { Which among the following is haloarene? } $$ 19. Which from following chemical activities does NOT exhibit good atom economy according to the principles of green chemist 20. Calculate activation energy for a reaction if it's rate doubles when temperature is raised from $20^{\circ} \mathrm{C}$ 21. What is the formal charge present on oxygen atom (numbered 1) in Lewis structure of $\mathrm{CO}_2$ ? 22. Which among the following is a monomer of natural rubber? 23. Identify the product obtained when alkyl alkanoate is hydrolysed with dilute HCl . 24. Which of the following changes exhibit that nitrogen undergoes oxidation?
25. The molar conductivity of $0.02 \mathrm{~mol} \mathrm{dm} \mathrm{dm}^{-3}$ solution of sodium hydroxide is $230.5 \Omeg 26. Calculate the volume occupied by particle in simple cubic unit cell if volume of unit cell is $5.5 \times 10^{-22} \math 27. Which of the following is primary benzylic alcohol? 28. Which from following elements is a decay product of thorium?
29. If half life of a first order reaction is 10 minutes, find the time required to decrease concentration of reactant from 30. Calculate the pOH of buffer solution formed from 0.3 M weak base and 0.45 M of its salt with strong acid $\left[\mathrm{ 31. Which from following complexes is having ambidentate ligand in it? 32. What is the minimum number of spheres required to develop an octahedral void?
33. How many moles of iodomethane are consumed in the following conversion?
$$ \mathrm{CH}_3 \mathrm{NH}_2 \xrightarrow[\Del 34. Identify the use of calcium carbonate from following. 35. Which of the following acid strength is used to get good yield of alkyl iodide from alcohol and sodium iodide in phospho 36. Identify false statement about transition elements. 37. Which from following terms is explained by first law of thermodynamics? 38. Calculate degree of dissociation of a weak monobasic acid in 0.01 M solution if dissociation constant is $1.6 \times 10^ 39. Which from following is an example of solution of solid as solute and liquid as solvent? 40. Identify the coordination number of aluminium in potassium trioxalatoaluminate(III). 41. Which of the following isomeric amines has the highest boiling point? 42. If $\mathrm{A}, \mathrm{B}, \mathrm{C}, \mathrm{D}$ are four different elements with outer electronic configuration as $ 43. What is the change in internal energy for $2 \mathrm{CO}_{(\mathrm{g})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow 2 \mathr 44. Calculate the final pressure required to reduce the volume of a gas to one third, if initial pressure is $1.6 \times 10^ 45. If osmotic pressure of 0.2 M aqueous glucose solution is 5 atm at 300 K . Calculate the concentration of glucose solutio 46. What is the IUPAC name of following compound?
47. Which amino acid from following contains highest number of N atoms in it? 48. Identify the reagent ' R ' necessary to bring the following conversion.
49. $$ \text { Identify ' } A \text { ' in the following reaction. } $$ 50. What are the possible values of magnetic quantum number for $p$ orbital?
Physics
1. Water is flowing through a horizontal pipe in a streamline flow. At the narrowest part of the pipe 2. Two different coils have self - inductance $\mathrm{L}_1=9 \mathrm{mH}$ and $\mathrm{L}_2=3 \mathrm{mH}$. The current in 3. Four masses of $1 \mathrm{~kg}, 2 \mathrm{~kg}, 3 \mathrm{~kg}$ and 4 kg are kept at co-ordinates $(0,0) \mathrm{m},(0,1 4. In between the plates of parallel plate capacitor of plate separation 'd' a dielectric plate of thickness ' $t$ ' is ins 5. A thin rod of length ' 4 L ' and mass ' 4 m ' is bent at the points as shown in the figure. The moment of inertia of the 6. The resistance of a coil for d.c. is $5 \Omega$. In a.c., the resistance will 7. When a ray of light is refracted from one medium to another, then the wavelength changes from $6000 \mathop {\rm{A}}\lim 8. A pipe closed at one end vibrating in fifth overtone is in unison with open pipe vibrating in its fifth overtone. The ra 9. Two stars 'A' and 'B' radiate maximum energy at $5200 \mathop {\rm{A}}\limits^ \circ$ and $6500 \mathop {\rm{A}}\limits^ 10. A hollow charged metal sphere has a radius ' $r$ '. If the potential difference between its surface and a point at a dis 11. For a given medium, the speed of light and the polarising angle are ' $v$ ' and ' $i_p$ ' respectively, then ( $\mathrm{ 12. A particle of mass ' m ' is executing S.H.M. about the origin on $x$-axis with frequency $\sqrt{\frac{\mathrm{ka}}{\pi \ 13. A satellite is revolving round the earth with orbital speed ' $\mathrm{V}_0$ '. If it stops suddenly, the speed with whi 14. The angular momentum of electron in hydrogen atom in first orbit is ' $L$ '. The change in angular momentum if electron 15. Four point masses, each of mass ' $m$ ' are arranged in $X-Y$ plane as shown in the figure. The moment of inertia of thi 16. In biprism experiment the maximum intensity is ' $\mathrm{I}_0$ '. If the path difference between the two interfering wa 17. Work done to get ' $n$ ' spherical drops of equal size from a single spherical drop of water, is proportional to 18. Diode and resistance are connected as shown in figure. Out of the following statements which one is TRUE?
19. Two uniform strings ' $A$ ' and ' $B$ ' made of steel are made to vibrate under same tension. If the first overtone of ' 20. Which one of the following is the correct equation for the electric circuit shown in the figure? 21. A pendulum is performing simple harmonic motion. The acceleration of the bob is $20 \mathrm{~cm} \mathrm{~s}^{-2}$ at a 22. A spherical body of radius ' $r$ ' radiates power ' $P$ ' at $T$ kelvin. If the radius $s$ halved and the temperature do 23. For a common emitter transistor configuration the ratio of $\frac{\mathrm{I}_{\mathrm{C}}}{\mathrm{I}_{\mathrm{E}}}=0.96 24. A stone is thrown upward with a speed 'u' from the top of a tower reaches the ground with velocity ' $3 u$ '. The height 25. A metal surface is illuminated by light of two different wavelengths 207 nm and 414 nm . The maximum speeds of photoelec 26. In thermodynamic process, which of the following statements is not true? 27. A charged particle is subjected to acceleration in a cyclotron which consists of two dees ' $\mathrm{D}_1$ ' and ' $D_2$ 28. In case of well of death which is a vertical cylindrical wall of radius ' $r$ ' inside which vehicle is driven in horizo 29. In an oscillating LC circuit, the maximum charge on the capacitor is ' $Q$ '. When the energy is stored equally between 30. Two particles execute S.H.M. of same amplitude and frequency along the same straight line path. They pass each other whe 31. The pitch of whistle of an engine appears to drop by $30 \%$ of the original value when it passes a stationary observer. 32. A solenoid is connected to a battery so that a steady current flows through it, If an iron core is inserted into the sol 33. A monoatomic ideal gas, initially at temperature ' $\mathrm{T}_1$ '' is enclosed in a cylinder fitted with a frictionles 34. In the diagram, the total electric flux through the closed surface ' S ' is
[Given $\mathrm{q}=$ charge
$\varepsilon_0=$ 35. If an electron in hydrogen atom jumps from $3^{\text {rd }}$ orbit to $2^{\text {nd }}$ orbit it emits a photon of wavel 36. A solenoid having 400 turns/metre has a core of a material with relative permeability 300 . If a current of 0.5 A is pas 37. The volume of a block of metal at $30^{\circ} \mathrm{C}$ changes by $0.12 \%$ when its temperature is increased to $70^ 38. A galvanometer of resistance ' $G$ ' can be converted into a voltmeter of range ( $0-\mathrm{V}$ ) volt by connecting a 39. A light metal disc of radius ' $r$ ' floats on water surface and bends the surface downwards along the perimeter making 40. The magnetic field at the centre of a current carrying circular coil of area ' $A$ ' is ' $B$ '. The magnetic moment of 41. A parallel combination of two capacitors of capacities ' C ' and ' $\frac{\mathrm{C}}{3}$, respectively is connected acr 42. A transparent sphere of refractive index ' $\mu$ ' and radius of curvature ' $R$ ' is kept in air. A point object is pla 43. The instantaneous value of current in an a.c. circuit is $I=2 \sin \left[100 \pi t+\frac{\pi}{3}\right]$ A. The current 44. A fixed mass of gas at constant pressure occupies a volume ' $V$ '. The gas undergoes a rise in temperature so that the 45. The Boolean expression for $\mathrm{X}-\mathrm{OR}$ gate $C=(A \oplus B)$ is equivalent to 46. The equation of simple harmonic progressive wave is given by $y=A \sin (100 \pi t-4 x)$. The distance between two partic 47. A photon and an electron have an equal energy ' E '. The ratio of wavelength ' $\lambda_{\mathrm{p}}$ ' of photon to tha 48. The height at which the acceleration due to gravity becomes $\frac{g}{4}$ in terms of $R$ is $[R=$ the radius of the ear 49. In a biprism experiment, fifth dark fringe is obtained at a point. A thin transparent film of refractive index ' $\mu$ ' 50. Figure shows a rectangular frame situated in a constant magnetic field. A wire BC of length 1 m is moved out with veloci
1
MHT CET (PCB) 2024 22th April Morning Shift
MCQ (Single Correct Answer)
+1
-0
Calculate the final pressure required to reduce the volume of a gas to one third, if initial pressure is $1.6 \times 10^5 \mathrm{Nm}^{-2}$.
A
$5.4 \times 10^5 \mathrm{Nm}^{-2}$
B
$1.6 \times 10^5 \mathrm{Nm}^{-2}$
C
$2.11 \times 10^5 \mathrm{Nm}^{-2}$
D
$4.8 \times 10^5 \mathrm{Nm}^{-2}$
2
MHT CET (PCB) 2024 22th April Morning Shift
MCQ (Single Correct Answer)
+1
-0
If osmotic pressure of 0.2 M aqueous glucose solution is 5 atm at 300 K . Calculate the concentration of glucose solution having osmotic pressure 2.5 atm at same temperature.
A
0.1 M
B
0.05 M
C
0.75 M
D
0.25 M
3
MHT CET (PCB) 2024 22th April Morning Shift
MCQ (Single Correct Answer)
+1
-0
What is the IUPAC name of following compound?
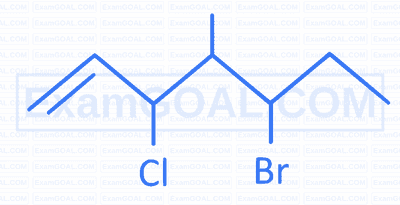
A
3-Chloro-4-methyl-5-bromohept-1-ene
B
4-Methyl-3-bromo-5-chlorohept-6-ene
C
3-Bromo-4-methyl-5-chlorohept-6-ene
D
5-Bromo-3-chloro-4-methylhept-1-ene
4
MHT CET (PCB) 2024 22th April Morning Shift
MCQ (Single Correct Answer)
+1
-0
Which amino acid from following contains highest number of N atoms in it?
A
Histidine
B
Lysine
C
Arginine
D
Glutamine
Paper analysis
Total Questions
Biology
100
Chemistry
50
Physics
50
More papers of MHT CET (Biology)
MHT CET (Biology)
Papers