1
JEE Advanced 2023 Paper 2 Online
Numerical
+3
-0

The entropy versus temperature plot for phases $\alpha$ and $\beta$ at 1 bar pressure is given. $S_{\mathrm{T}}$ and $S_0$ are entropies of the phases at temperatures $\mathrm{T}$ and $0 \mathrm{~K}$, respectively.

The transition temperature for $\alpha$ to $\beta$ phase change is $600 \mathrm{~K}$ and $C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}=1 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$. Assume $\left(C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}\right)$ is independent of temperature in the range of 200 to $700 \mathrm{~K} . C_{\mathrm{p}, \alpha}$ and $C_{\mathrm{p}, \beta}$ are heat capacities of $\alpha$ and $\beta$ phases, respectively.

The transition temperature for $\alpha$ to $\beta$ phase change is $600 \mathrm{~K}$ and $C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}=1 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$. Assume $\left(C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}\right)$ is independent of temperature in the range of 200 to $700 \mathrm{~K} . C_{\mathrm{p}, \alpha}$ and $C_{\mathrm{p}, \beta}$ are heat capacities of $\alpha$ and $\beta$ phases, respectively.
The value of entropy change, $S_\beta-S_\alpha$ (in $\mathrm{J} \mathrm{mol}^{-1} \mathrm{~K}^{-1}$ ), at $300 \mathrm{~K}$ is _______.
[Use : $\ln 2=0.69$
Given : $S_\beta-S_\alpha=0$ at $0 \mathrm{~K}$ ]
[Use : $\ln 2=0.69$
Given : $S_\beta-S_\alpha=0$ at $0 \mathrm{~K}$ ]
Your input ____
2
JEE Advanced 2023 Paper 2 Online
Numerical
+3
-0

The entropy versus temperature plot for phases $\alpha$ and $\beta$ at 1 bar pressure is given. $S_{\mathrm{T}}$ and $S_0$ are entropies of the phases at temperatures $\mathrm{T}$ and $0 \mathrm{~K}$, respectively.

The transition temperature for $\alpha$ to $\beta$ phase change is $600 \mathrm{~K}$ and $C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}=1 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$. Assume $\left(C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}\right)$ is independent of temperature in the range of 200 to $700 \mathrm{~K} . C_{\mathrm{p}, \alpha}$ and $C_{\mathrm{p}, \beta}$ are heat capacities of $\alpha$ and $\beta$ phases, respectively.

The transition temperature for $\alpha$ to $\beta$ phase change is $600 \mathrm{~K}$ and $C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}=1 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$. Assume $\left(C_{\mathrm{p}, \beta}-C_{\mathrm{p}, \alpha}\right)$ is independent of temperature in the range of 200 to $700 \mathrm{~K} . C_{\mathrm{p}, \alpha}$ and $C_{\mathrm{p}, \beta}$ are heat capacities of $\alpha$ and $\beta$ phases, respectively.
$$
\text { The value of enthalpy change, } \mathrm{H}_\beta-\mathrm{H}_\alpha \text { (in } \mathrm{J} \mathrm{mol}^{-1} \text { ), at } 300 \mathrm{~K} \text { is }
$$ ________.
Your input ____
3
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

One mole of an ideal monoatomic gas undergoes two reversible processes $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ as shown in the given figure:
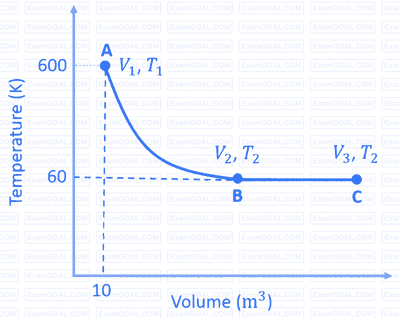
$\mathrm{A} \rightarrow \mathrm{B}$ is an adiabatic process. If the total heat absorbed in the entire process $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ is $\mathrm{R} T_2 \ln 10$, the value of $2 \log V_3$ is _______.
[Use, molar heat capacity of the gas at constant pressure, $C_{\mathrm{p}, \mathrm{m}}=\frac{5}{2} \mathrm{R}$ ]

$\mathrm{A} \rightarrow \mathrm{B}$ is an adiabatic process. If the total heat absorbed in the entire process $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ is $\mathrm{R} T_2 \ln 10$, the value of $2 \log V_3$ is _______.
[Use, molar heat capacity of the gas at constant pressure, $C_{\mathrm{p}, \mathrm{m}}=\frac{5}{2} \mathrm{R}$ ]
Your input ____
4
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

In a one-litre flask, 6 moles of $A$ undergoes the reaction $A(\mathrm{~g}) \rightleftharpoons P(\mathrm{~g})$. The progress of product formation at two temperatures (in Kelvin), $\mathrm{T}_1$ and $\mathrm{T}_2$, is shown in the figure:

If $\mathrm{T}_1=2 \mathrm{~T}_2$ and $\left(\Delta \mathrm{G}_2^{\Theta}-\Delta \mathrm{G}_1^{\Theta}\right)=\mathrm{RT}_2 \ln \mathrm{x}$, then the value of $\mathrm{x}$ is _______.
$\left[\Delta \mathrm{G}_1^{\Theta}\right.$ and $\Delta \mathrm{G}_2^{\Theta}$ are standard Gibb's free energy change for the reaction at temperatures $\mathrm{T}_1$ and $\mathrm{T}_2$, respectively.]

If $\mathrm{T}_1=2 \mathrm{~T}_2$ and $\left(\Delta \mathrm{G}_2^{\Theta}-\Delta \mathrm{G}_1^{\Theta}\right)=\mathrm{RT}_2 \ln \mathrm{x}$, then the value of $\mathrm{x}$ is _______.
$\left[\Delta \mathrm{G}_1^{\Theta}\right.$ and $\Delta \mathrm{G}_2^{\Theta}$ are standard Gibb's free energy change for the reaction at temperatures $\mathrm{T}_1$ and $\mathrm{T}_2$, respectively.]
Your input ____
Questions Asked from Thermodynamics (Numerical)
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2023 Paper 2 Online (2)
JEE Advanced 2023 Paper 1 Online (2)
JEE Advanced 2022 Paper 1 Online (1)
JEE Advanced 2021 Paper 2 Online (1)
JEE Advanced 2021 Paper 1 Online (2)
JEE Advanced 2020 Paper 2 Offline (1)
JEE Advanced 2020 Paper 1 Offline (1)
JEE Advanced 2018 Paper 2 Offline (1)
IIT-JEE 2009 Paper 2 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Equilibrium Solutions States of Matter Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State & Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra and 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus